MHC II Antibodies
Background
MHC II molecules are heterodimer membrane proteins composed of two chains, α and β, and are widely distributed on the surface of antigen-presenting cells. This type of molecule specifically recognizes and binds to exogenous antigenic peptides, presenting them to CD4+ T lymphocytes and thereby activating adaptive immune responses. Its core role in immune regulation was first clarified by scholars such as Baruj Benacerraf, and the related research won the Nobel Prize in Physiology or Medicine in 1980. The MHC II gene cluster has extremely high polymorphism. This genetic diversity directly affects an individual's response ability to pathogens and susceptibility to autoimmune diseases. The precise interaction mechanism between its three-dimensional structure and antigenic peptides has become a research paradigm in immunology, profoundly promoting breakthroughs in vaccine development, organ transplantation matching, and the treatment of autoimmune diseases.
Structure of MHC II
MHC II molecules are heterodimers composed of two transmembrane polypeptide chains, α and β, with a molecular weight of approximately 25-30 kDa. The specific value may fluctuate slightly due to locus differences (such as HLA-DR, DP, DQ) and species variations. The MHC II molecules of different species have significant polymorphisms in the amino acid sequences of the antigen-binding slots, which directly determines the specificity of their presentation of exogenous antigenic peptides.
| Species | Human | Mouse | Rhesus monkey | Pig |
| Molecular Weight (kDa) | ~33-35 / ~27-29 | ~33-35 / ~27-29 | ~33-35 / ~27-29 | ~32-34 / ~27-29 |
| Primary Structural Differences | Highly polymorphic, encoded by HLA-DR/DQ/DP genes | Highly polymorphic, encoded by the H-2-A/H-2-E gene | Highly homologous to humans | Has a unique SLA allelic diversity |
The primary structure of this protein is composed of the α1, α2, β1, and β2 domains. Among them, the α1 and β1 domains at the distal end of the membrane jointly fold to form an open antigenic peptide binding slot, which is the core of its molecular function. Its secondary structure is mainly composed of anti-parallel β sheets as the base of the bonding groove, with α -helices serving as the side walls on both sides. Key peptide-binding residues (such as Hisβ81 and Tyrβ78 in HL-DR1) precisely anchorage the two ends of antigenic peptides through hydrogen bonds and hydrophobic interactions, while T-cell receptors specifically recognize the complex epitopes jointly formed by MHC II and antigenic peptides, thereby initiating adaptive immune responses.
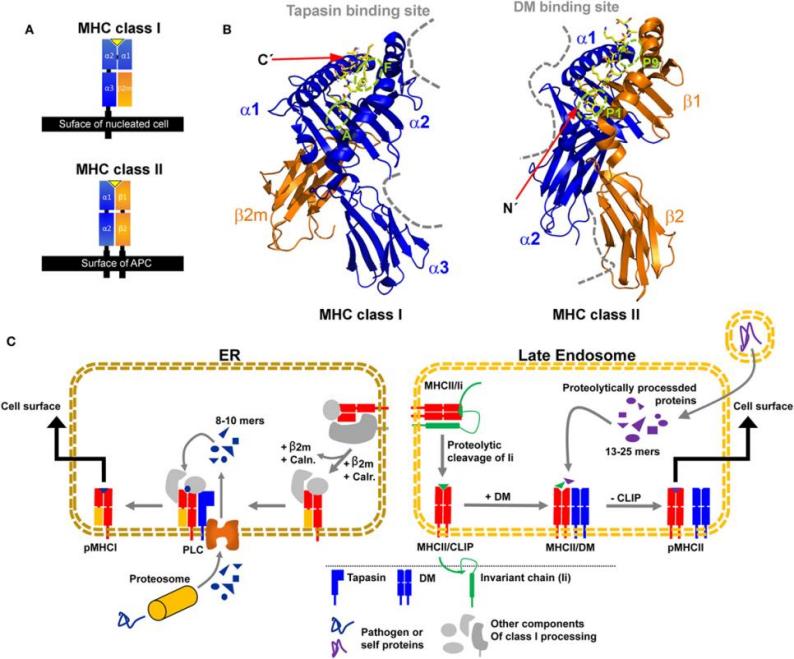 Fig. 1 Structural characteristics of major histocompatibility complex (MHC) class I and MHC class II proteins and their compartment-dependent loading with processed peptides.1
Fig. 1 Structural characteristics of major histocompatibility complex (MHC) class I and MHC class II proteins and their compartment-dependent loading with processed peptides.1
Key structural properties of MHC II:
- Membrane surface heterodimeric structure consisting of α and β strands
- Open antigen-peptide binding grooves are formed at the distal end of the membrane
- Combined with open on both ends of the tank, can hold a long exogenous antigen peptide
Functions of MHC II
The core function of MHC II molecules is to present exogenous antigens to activate CD4+ T cells. However, it is also deeply involved in a variety of key physiological and pathological processes such as immune regulation, autoimmune tolerance and pathogen defense.
| Function | Description |
| Presentation of exogenous antigens | MHC II molecules on the surface of professional antigen-presenting cells (such as dendritic cells and macrophages) bind to exogenous antigen peptides that have been phagocytosed and processed, and present them to CD4+ T cells. |
| Activation of CD4+ T cells | The T-cell receptor (TCR) recognizes the antigenic peptide-MHC II complex, providing activation signals and initiating adaptive immune responses. |
| Immune regulation | By regulating the specificity and intensity of antigen presentation, it directly affects the differentiation of different helper T cell subsets such as Th1, Th2, and Treg, thereby regulating the direction of the immune response. |
| Maintenance of autoimmune tolerance | In the thymus, immature T cells undergo negative selection by coming into contact with MHC II molecules filled with autoantigen peptides, eliminating autoreactive T cells and preventing autoimmune diseases. |
| Pathogen immune response | The presentation of antigenic peptides from different pathogens (such as bacteria and viruses) is a crucial first step in clearing extracellular infections and initiating humoral immunity. |
The binding of MHC II molecules to antigenic peptides is broad-spectrum but selective. The polymorphic amino acid residues in its binding slots determine the specificity of the peptide's anchored residues. This characteristic leads to natural differences in the immune response ability of different individuals to the same pathogen.
Applications of MHC II and MHC II Antibody in Literature
1. Wieczorek, Marek, et al. "Major histocompatibility complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation." Frontiers in immunology 8 (2017): 292. https://doi.org/10.3389/fimmu.2017.00292
The article indicates that MHC class II molecules affect the presentation of antigenic peptides through dynamic conformational changes, and the process is catalyzed by HLA-DM editing, but the specific mechanism remains controversial. The sensitivity differences of different alleles to peptide editing are related to protein intermediate states, which may regulate immune dominance and the occurrence of autoimmune diseases.
2. Santambrogio, Laura. "Molecular determinants regulating the plasticity of the MHC class II immunopeptidome." Frontiers in Immunology 13 (2022): 878271. https://doi.org/10.3389/fimmu.2022.878271
In recent years, studies on the MHC II class molecular ligand group have revealed the regulatory roles of polymorphism, endosome processing differences, and molecular chaperone DM/DO, jointly shaping the diversity and dynamics of the MHC II presenting peptide group, enabling it to sensitively reflect microenvironmental changes and activate CD4 T cell responses.
3. Fooksman, David R. "Organizing MHC class II presentation." Frontiers in immunology 5 (2014): 158. https://doi.org/10.3389/fimmu.2014.00158
The article indicates that the tissue distribution and dynamic diffusion of MHC class II molecules on antigen-presenting cell membranes affect the activation of CD4+ T cells. Their interaction with lipid rafts and other membrane proteins may regulate the formation of immune synapses, but the specific mechanism still awaits in-depth study.
4. Yi, Ruibin, et al. "MHC-II signature correlates with anti-tumor immunity and predicts anti-PD-L1 response of bladder cancer." Frontiers in Cell and Developmental Biology 10 (2022): 757137. https://doi.org/10.3389/fcell.2022.757137
This study established the MHC-II characteristic scoring system for the first time through the ssGSEA algorithm and found that it could independently predict the efficacy and prognosis of immunotherapy for bladder cancer, which was superior to the tumor mutation burden. The high MHC-II characteristic group was characterized by increased immune cell infiltration and immune activation status, while the low characteristic group showed metabolic reprogramming, with TP53 and FGFR3 gene variations playing a key role.
5. Leung, Carol SK. "Endogenous antigen presentation of MHC class II epitopes through non-autophagic pathways." Frontiers in immunology 6 (2015): 464. https://doi.org/10.3389/fimmu.2015.00464
In this study, in addition to presenting exogenous antigens, MHC class II molecules can also present endogenous antigens through non-autophagic pathways (including MHC class I pathways, HSP90-mediated pathways, and plasma membrane internalization, etc.), expanding their presentation mechanism for intracellular proteins.
Creative Biolabs: MHC II Antibodies for Research
Creative Biolabs specializes in the production of high-quality MHC II antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MHC II Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MHC II antibodies, custom preparations, or technical support, contact us at email.
Reference
- Wieczorek, Marek, et al. "Major histocompatibility complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation." Frontiers in immunology 8 (2017): 292. https://doi.org/10.3389/fimmu.2017.00292
Anti-MHC II antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CCDC25 Recombinant Antibody (CBLC132-LY) (CBMAB-C9786-LY)

-
Mouse Anti-Acetyl-α-Tubulin (Lys40) Recombinant Antibody (V2-623485) (CBMAB-CP2897-LY)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-1728) (CBMAB-2077-YY)

-
Mouse Anti-AQP2 Recombinant Antibody (G-3) (CBMAB-A3359-YC)

-
Mouse Anti-BCL2L1 Recombinant Antibody (H5) (CBMAB-1025CQ)

-
Mouse Anti-ADIPOR2 Recombinant Antibody (V2-179983) (CBMAB-A1369-YC)

-
Mouse Anti-CD83 Recombinant Antibody (HB15) (CBMAB-C1765-CQ)

-
Mouse Anti-BACE1 Recombinant Antibody (CBLNB-121) (CBMAB-1180-CN)

-
Mouse Anti-FLI1 Recombinant Antibody (CBXF-0733) (CBMAB-F0435-CQ)

-
Mouse Anti-BMI1 Recombinant Antibody (CBYC-P026) (CBMAB-P0108-YC)

-
Mouse Anti-FLT1 Recombinant Antibody (11) (CBMAB-V0154-LY)

-
Mouse Anti-C4B Recombinant Antibody (CBYY-C2996) (CBMAB-C4439-YY)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

-
Mouse Anti-CTNND1 Recombinant Antibody (CBFYC-2414) (CBMAB-C2487-FY)

-
Mouse Anti-BSN Recombinant Antibody (219E1) (CBMAB-1228-CN)

-
Mouse Anti-AKT1 (Phosphorylated S473) Recombinant Antibody (V2-505430) (PTM-CBMAB-0067LY)

-
Mouse Anti-CA9 Recombinant Antibody (CBXC-2079) (CBMAB-C0131-CQ)

-
Mouse Anti-CD24 Recombinant Antibody (2Q1282) (CBMAB-C1624-CN)

-
Mouse Anti-AKR1B1 Antibody (V2-2449) (CBMAB-1001CQ)

-
Mouse Anti-AKT1 Recombinant Antibody (V2-180546) (CBMAB-A2070-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








