RARB Antibodies
Background
RARB is an important tumor suppressor protein, mainly existing in the nuclei of eukaryotes. It regulates the cell cycle process and inhibits abnormal proliferation by binding to the E2F family of transcription factors, thereby maintaining genomic stability. During the development of the retina, RARB plays a key role in the differentiation of photoreceptor cells, and its functional deficiency can lead to the occurrence of malignant tumors such as retinoblastoma. This protein was first discovered by the Weinberg team in 1987 when they were studying retinoblastoma. Its gene was the first identified tumor suppressor gene, and this discovery brought a major breakthrough to the field of cancer research. The three-dimensional structural analysis of RARB provides an important basis for understanding the regulatory mechanism of the cell cycle and promotes the research and development of targeted anti-cancer drugs. Due to its core biological functions, RARB remains a key focus in cell cycle, cancer treatment and epigenetic research to this day.
Structure of RARB
RARB is a medium-sized nucleoprotein with a molecular weight of approximately 48 kDa. The molecular weight of this protein varies slightly among different species, which mainly results from the conjunctive variations of its amino acid sequence.
| Species | Human | Mice | Rats | Zebrafish | Fruit flies |
| Molecular Weight (kDa) | 48.2 | 47.8 | 48.1 | 45.3 | 42.6 |
| Primary Structural Differences | Contains DNA binding domain and ligand binding domain | The transcriptional activation domain with 95% homology to humans is slightly different | Transcriptional activation domain (A/B area) is slightly different | Structure is simplified, and retain the core functional domains, but part of the lack of regulatory regions | Only the most core functional domains are retained, lacking certain regulatory elements of mammalian RARBs |
RARB protein consists of 456 amino acids and has typical structural features of nuclear receptors. This protein recognizes specific DNA reaction elements by binding to retinoic acid to form heterodimers. The cysteine residues in its DNA binding domain coordinate with zinc ions to form a stable zinc finger structure, which is crucial for gene regulation. The ligand-binding domain of RARB has a typical nuclear receptor folding pattern, consisting of 12 α -helices forming a hydrophobic ligand-binding pocket. The conformational change of helix H12 determines whether the co-activator is recruited or not.
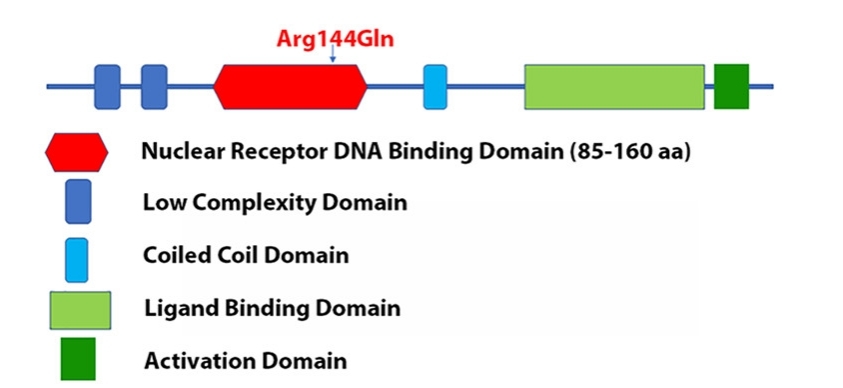 Fig. 1 Mutation localization of Arg144Gln in the DNA binding domain of RARB protein.1
Fig. 1 Mutation localization of Arg144Gln in the DNA binding domain of RARB protein.1
Key structural properties of RARB:
- Modular nuclear receptor structure (A-F functional domain)
- Double zinc refers to the DNA binding domain (Region C)
- Ligand-binding pocket (composed of 12 α -helices)
- Conformational changes of the H12 helix induced by retinoic acid binding
- Dimerization interface (binding to RXR receptors)
Functions of RARB
The core function of RARB protein is to participate in the cell cycle regulation and differentiation process as a transcriptional regulatory factor. Furthermore, it plays an important role in a variety of physiological and pathological processes, including tumor suppression and developmental regulation.
| Function | Description |
| Cell cycle regulation | By binding to the E2F transcription factor, it inhibits the expression of key genes in the cell cycle and prevents abnormal cell proliferation. |
| Tumor suppressive effect | As a tumor suppressor gene in various cancers such as retinoblastoma, its deletion can lead to tumorigenesis. |
| Developmental regulation | Regulate cell differentiation during embryonic development, especially the development of the nervous system and retina. |
| Retinoic acid signal transduction | As a retinoic acid receptor, it mediates the regulation of retinoic acid on gene expression and affects the cell differentiation process. |
| Epigenetic regulation | By recruiting modification factors such as histone deacetylase (HDAC), the chromatin structure and gene expression profile are altered. |
RARB protein DNA binding properties show the typical nuclear receptor response element identification model, combined with the synergy of hemoglobin, RARB with RXR form different source dimers to enhance its target genes to identify specific and control accuracy. This mechanism enables it to precisely regulate the downstream gene network and play a key role in development and diseases.
Applications of RARB and RARB Antibody in Literature
1. Hinteregger, Barbara, et al. "Transgene integration causes RARB downregulation in homozygous Tg4–42 mice." Scientific reports 10.1 (2020): 6377. https://doi.org/10.1038/s41598-020-63512-8
This article indicates that the Tg4-42 transgenic mouse model study found that the deletion of exon 2 of the RARB gene in homozygous mice led to a significant decrease in protein expression, which may affect their phenotype. However, the RARB level of heterozygous mice was normal, and the phenotype was only caused by the expression of Aβ4-42. The research suggests that when establishing disease models, it is necessary to confirm the transgenic integration sites through sequencing technology to avoid unexpected genetic influences.
2. Replogle, Maria R., et al. "A De Novo Noncoding RARB Variant Associated with Complex Microphthalmia Alters a Putative Regulatory Element." Human mutation 2024.1 (2024): 6619280. https://doi.org/10.1155/2024/6619280
This article indicates that A novel variation c.157+1895G>A was found in the intron region of the RARB gene in patients with complex microphthalmia. Functional experiments confirmed that this variation did not affect mRNA splicing, but could enhance the promoter activity and lead to overexpression of RARB. Overexpressed RARB promotes cell proliferation and upregulates the downstream gene FOXC1, which may cause abnormal ocular development.
3. Huang, Linhui, et al. "RARB genetic variants might contribute to the risk of chronic obstructive pulmonary disease based on a case-control study." Annals of Medicine 57.1 (2025): 2445195. https://doi.org/10.1080/07853890.2024.2445195
This article indicates that the polymorphism of the RARB gene may affect the progression of COPD by regulating inflammatory responses and apoptosis. This study explored for the first time the association between RARB single nucleotide polymorphisms (SNPs) and the susceptibility to COPD, providing new clues for revealing the pathogenesis of COPD.
4. Danos, Pierina, et al. "Promoter hypermethylation of RARB and GSTP1 genes in plasma cell-free DNA as breast cancer biomarkers in Peruvian women." Molecular Genetics & Genomic Medicine 11.12 (2023): e2260. https://doi.org/10.1002/mgg3.2260
This article indicates that hypermethylation of the promoters of the RARB and GSTP1 genes is significantly associated with breast cancer in the Peruvian population. This study employed the liquid biopsy method to analyze the relationship between the methylation levels of these two tumor suppressor genes and the clinicopathological characteristics of breast cancer.
Creative Biolabs: RARB Antibodies for Research
Creative Biolabs specializes in the production of high-quality RARB antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for Western blot, immunohistochemistry, flow cytometry, ELISA, and other diagnostic methodologies.
- Custom RARB Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our RARB antibodies, custom preparations, or technical support, contact us at info@creative-biolabs.com.
Reference
- Kalaskar, Vijay K., et al. "High-throughput custom capture sequencing identifies novel mutations in coloboma-associated genes: Mutation in DNA-binding domain of retinoic acid receptor beta affects nuclear localization causing ocular coloboma." Human mutation 41.3 (2020): 678-695. https://doi.org/10.1002/humu.23954
Anti-RARB antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CECR2 Recombinant Antibody (CBWJC-2465) (CBMAB-C3533WJ)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-CASQ1 Recombinant Antibody (CBFYC-0863) (CBMAB-C0918-FY)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Rat Anti-CD34 Recombinant Antibody (MEC 14.7) (CBMAB-C10196-LY)

-
Rabbit Anti-CCL5 Recombinant Antibody (R0437) (CBMAB-R0437-CN)

-
Mouse Anti-CEMIP Recombinant Antibody (3C12) (CBMAB-K0296-LY)

-
Mouse Anti-ADGRL2 Recombinant Antibody (V2-58519) (CBMAB-L0166-YJ)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-8-oxoguanine Recombinant Antibody (V2-7719) (CBMAB-1898CQ)

-
Mouse Anti-BIRC5 Recombinant Antibody (6E4) (CBMAB-CP2646-LY)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

-
Mouse Anti-ALOX5 Recombinant Antibody (33) (CBMAB-1890CQ)

-
Mouse Anti-CD2AP Recombinant Antibody (BR083) (CBMAB-BR083LY)

-
Mouse Anti-AP4E1 Recombinant Antibody (32) (CBMAB-A2996-YC)

-
Mouse Anti-DMD Recombinant Antibody (D1190) (CBMAB-D1190-YC)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

-
Mouse Anti-Acetyl SMC3 (K105/K106) Recombinant Antibody (V2-634053) (CBMAB-AP052LY)

-
Rabbit Anti-ADRA1A Recombinant Antibody (V2-12532) (CBMAB-1022-CN)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








