TBCC Antibodies
Background
TBCC gene encoding protein is a kind of microtubule binding protein, mainly involved in cytoskeletal microtubules network dynamic assembly and stability. This protein regulates spindle formation and mitosis by interacting with tubulin, playing a crucial role in maintaining cell morphology and the correct separation of chromosomes. Research has found that TBCC is abnormally expressed in various cancers and may be closely related to the proliferation and metastasis of tumor cells. This gene was first identified in 1994. The three-dimensional structure analysis of its encoded protein revealed a unique microtubule depolymerization mechanism, providing a new idea for the development of anti-cancer drugs targeting microtubules. As an important component of the cell cycle regulatory network, the functional research of TBCC proteins has greatly promoted people's understanding of cell division, microtubule dynamics, and the molecular mechanisms of related diseases.
Structure of TBCC
TBCC is a microtubule-binding protein with a molecular weight of approximately 55 kDa, and there are slight differences among different species:
| Species | Human | Mouse | Zebrafish | Fruit Fly |
| Molecular Weight (kDa) | 55 | 54.8 | 54.5 | 53.7 |
| Primary Structural Differences | Highly conserved microtubule binding domains | Highly homologous to humans | With similar functions but sequence differences | Simplify the structure, function similar to |
The TBCC protein is composed of approximately 500 amino acids and its structure encompasses multiple functional domains, among which the core β -propeller structure is responsible for binding to tubulin. This protein affects cell division and morphological maintenance by regulating the depolymerization and recombination of microtubules. The key amino acids in its active center (such as conserved Glu residues) are crucial to microtubule kinetics. The tertiary structure of TBCC presents a typical spherical conformation, stabilizing the microtubule binding interface through charge interaction, while the C-terminal flexible region participates in the interaction with other cytoskeletal regulatory factors.
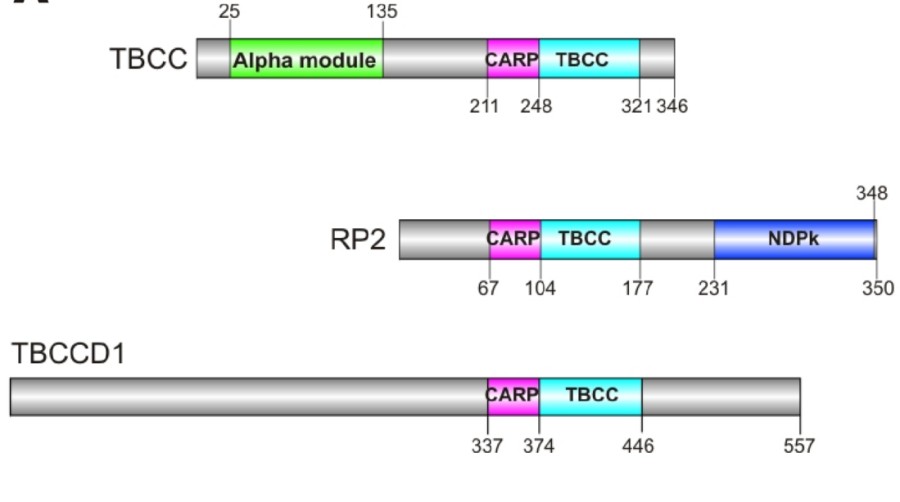 Fig. 1 Human TBCC protein family.1
Fig. 1 Human TBCC protein family.1
Key structural properties of TBCC:
- β -propeller domain
- Conserved acidic amino acid clusters
- Flexible C-end domain
Functions of TBCC
The main function of the protein encoded by the TBCC gene is to regulate microtubule dynamics and cell division, while also participating in a variety of cellular physiological processes:
| Function | Description |
| Regulation of microtubule depolymerization | The dynamic balance of the cytoskeleton is maintained by selectively depolymerizing microtubules through the β -propeller domain. |
| Mitotic regulation | Participate in spindle assembly and chromosome separation to ensure the normal progress of the cell cycle. |
| Regulation of cell migration | By influencing the recombination of microtubule networks, it participates in the establishment of cell polarity and motility. |
| Tumorigenesis association | The expression is abnormal in a variety of cancers and is associated with tumor cell invasion and metastasis. |
| Cilia/flagellum assembly | Involved in matrix microtubule organization, affecting ciliary structure and function. |
The mechanism of action of TBCC protein is ATP-dependent, and its activity is strictly regulated by phosphorylation modifications (such as CDK1-mediated Ser89 phosphorylation). When forming a complex with the microtubule-binding protein TBCB, its depolymerization activity is significantly enhanced, indicating its core role in microtubule remodeling. The expression level of this protein increases in rapidly proliferating cells, suggesting its close association with the cell cycle process.
Applications of TBCC and TBCC Antibody in Literature
1. Guo, Yaru, et al. "TBCC Domain-Containing Protein Regulates Sporulation and Virulence of Phytophthora capsici via Nutrient-Responsive Signaling." International Journal of Molecular Sciences 25.22 (2024): 12301.https://doi.org/10.3390/ijms252212301
In this study, through transcriptomics and CRISPR-Cas9 technology, it was found that the pathogenic factor PcTBCC1 of Oomycetes (containing tubulin binding domain and chloroplast targeted peptide) was significantly upregulated in host interactions. After gene knockout, the formation, differentiation and pathogenicity of pathogenic bacteria spores were significantly weakened, and they were sensitive to osmotic stress, indicating that TBCC is a conserved pathogenic determinant and can be a potential target for ovulatory drugs.
2. Hage-Sleiman, Rouba, et al. "Tubulin binding cofactor C (TBCC) suppresses tumor growth and enhances chemosensitivity in human breast cancer cells." BMC cancer 10.1 (2010): 135.https://doi.org/10.1186/1471-2407-10-135
In this study, by regulating the expression of tubulin chaperone TBCC in breast cancer cells, it was found that overexpression of TBCC led to a reduction in polymerizable tubulin, a decrease in microtubule dynamics, and a delay in the G2/M phase of the cell cycle. In vivo experiments have shown that high expression of TBCC inhibits tumor growth and simultaneously enhances the sensitivity of cancer cells to microtubule drugs, suggesting that TBCC is a key factor in regulating tumor phenotypes and chemotherapy responses.
3. Garcia-Mayoral, Mª Flor, et al. "The solution structure of the N-terminal domain of human tubulin binding cofactor C reveals a platform for tubulin interaction." PloS one 6.10 (2011): e25912.https://doi.org/10.1371/journal.pone.0025912
Research has found that tubulin chaperone (TBCC) interacts with tubulin through its N-terminal disordered region and participates in spindle formation. The absence of TBCC leads to abnormal multipolar spindles and mitosis. NMR analysis revealed that the N-terminal of TBCC had a spectrin-like structure, and its 30-amino acid flexible fragment directly mediated tubulin recognition, providing new insights into the TBCC-Tubulin interaction mechanism.
4. Barrack, Keri L., et al. "Crystal structure of the C-terminal domain of tubulin-binding cofactor C from Leishmania major." Molecular and Biochemical Parasitology 201.1 (2015): 26-30. https://doi.org/10.1016/j.molbiopara.2015.05.003
This study resolved the C-terminal domain of Leishmania TBCC protein (with A resolution of 2.2 A) and found that it presented a triangular prism structure formed by five right-handed β -helices. Structural comparison indicates that this β -helical surface may be involved in the interaction of the β-tubulin:GTP complex, revealing the molecular basis by which TBCC promotes GTPase activity.
5. Taheri, Aryan, et al. "Cryo-EM structures of the tubulin cofactors reveal the molecular basis for the biogenesis of alpha/beta-tubulin." bioRxiv (2024): 2024-01.https://doi.org/10.1101/2024.01.29.577855
In this study, the structure of the TBC-DEG-TBCC complex and αβ-tubulin was resolved by cryo-electron microscopy. It was found that TBCC pulled the C-terminal of α-tubulin by rotating the "lever arm" of TBCE clockwise, while TBCD fixed β-tubulin. The "vise" molecular mechanism of GTP hydrolysis-dependent tubulin assembly was revealed.
Creative Biolabs: TBCC Antibodies for Research
Creative Biolabs specializes in the production of high-quality TBCC antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom TBCC Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our TBCC antibodies, custom preparations, or technical support, contact us at email.
Reference
- Garcia-Mayoral, Mª Flor, et al. "The solution structure of the N-terminal domain of human tubulin binding cofactor C reveals a platform for tubulin interaction." PloS one 6.10 (2011): e25912.https://doi.org/10.1371/journal.pone.0025912
Anti-TBCC antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-EGR1 Recombinant Antibody (CBWJZ-100) (CBMAB-Z0289-WJ)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Mouse Anti-BrdU Recombinant Antibody (IIB5) (CBMAB-1038CQ)

-
Mouse Anti-ADAM29 Recombinant Antibody (V2-179787) (CBMAB-A1149-YC)

-
Mouse Anti-AGK Recombinant Antibody (V2-258056) (CBMAB-M0989-FY)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Mouse Anti-ENO2 Recombinant Antibody (85F11) (CBMAB-0276CQ)

-
Rat Anti-ABCC11 Recombinant Antibody (V2-179001) (CBMAB-A0236-YC)

-
Mouse Anti-ALDOA Recombinant Antibody (A2) (CBMAB-A2316-YC)

-
Mouse Anti-CECR2 Recombinant Antibody (CBWJC-2465) (CBMAB-C3533WJ)

-
Mouse Anti-ALPL Antibody (B4-78) (CBMAB-1009CQ)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-CD46 Recombinant Antibody (CBFYC-0076) (CBMAB-C0085-FY)

-
Mouse Anti-CTNND1 Recombinant Antibody (CBFYC-2414) (CBMAB-C2487-FY)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Rabbit Anti-ENO2 Recombinant Antibody (BA0013) (CBMAB-0272CQ)

-
Rat Anti-AChR Recombinant Antibody (V2-12500) (CBMAB-0990-CN)

-
Rabbit Anti-CCL5 Recombinant Antibody (R0437) (CBMAB-R0437-CN)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








