TIMP1 Antibodies
Background
TIMP1 is a secretory glycoprotein that mainly exists in the extracellular matrix and body fluids of vertebrates, serving as a key inhibitor of matrix metalloproteinases (MMPs). The protein encoded by this gene maintains tissue homeostasis and prevents excessive degradation of the extracellular matrix by specifically binding to MMPs and blocking their activity. In the process of tissue repair and immune regulation, TIMP1 plays a core role by regulating the balance of protease networks. This gene was first identified in 1985 and is the first member of the family of metalloproteinase inhibitors to be discovered. The study of its three-dimensional structure has revealed typical "wedge-shaped" inhibitory domain characteristics. This molecular mechanism, which combines inhibitory functions with cellular signal regulation capabilities, has become an important model for studying fibrotic diseases and the tumor microenvironment, promoting the understanding of the role of protein-protein interaction networks in pathophysiological processes.
Structure of TIMP1
TIMP1 is a secretory glycoprotein with a molecular weight of approximately 28.4 kDa. Its actual measured value usually fluctuates within the range of 28-34 kDa, and this difference is mainly due to different degrees of glycosylation modification.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 28.4 | 28.2 | 28.3 | 28.5 |
| Primary Structural Differences | Contains 12 conserved cysteines forming 6 pairs of disulfide bonds | The N-glycosylation site is highly homologous to humans | The C-terminal domain is specifically related to the binding of MMP | Degree of glycosylation interspecific differences |
This protein is composed of 207 amino acids. Its primary structure is stably folded by six pairs of highly conserved disulfide bonds, forming two typical independent domains at the N-terminal and C-terminal. The N-terminal domain forms a compact three-dimensional conformation, containing active sites that specifically bind to the catalytic domain of the matrix metalloproteinase, and the inhibitory function is achieved through the zinc ion-binding motif. The C-terminal domain presents a unique β -barrel folding, participating in the formation of protein polymers and the regulation of cell signals.
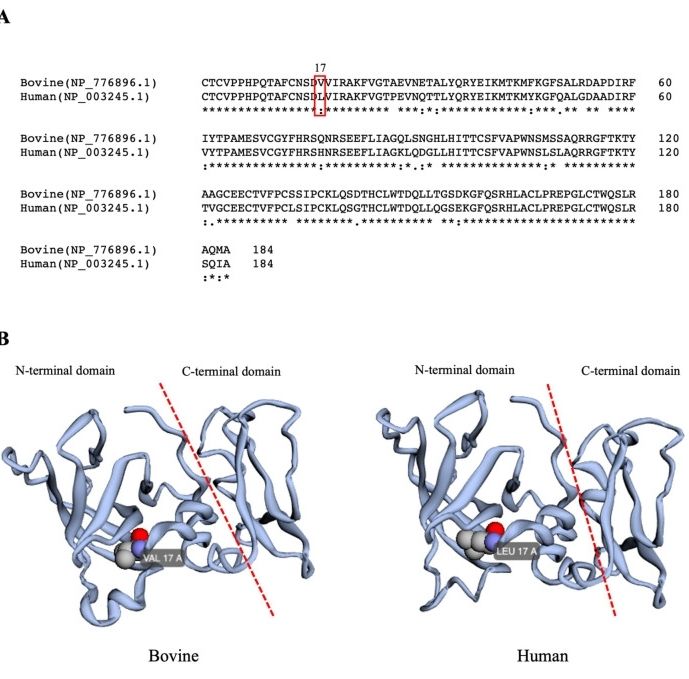 Fig. 1 A comparison of bovine and human TIMP1 proteins.1
Fig. 1 A comparison of bovine and human TIMP1 proteins.1
Key structural properties of TIMP1:
- Conserved disulfide bond topology (6 pairs of disulfide bonds are stably folded)
- N-terminal domain structure formation of MMPS inhibitory activity center
- The C-terminal domain presents a unique β -barrel folded conformation
Functions of TIMP1
The core function of the TIMP1 gene-encoded protein is to inhibit matrix metalloproteinases to maintain extracellular matrix homeostasis, while also participating in various physiological and pathological processes.
| Function | Description |
| Protease inhibition | By specifically binding to the MMP catalytic center through the N-terminal domain, the degradation of matrix components such as collagen and gelatin is irreversibly blocked. |
| Regulation of tissue remodeling | During the process of wound healing and tissue repair, precisely balance the synthesis and catabolism of extracellular matrix. |
| Cell proliferation promotion | Activate specific transmembrane receptor-mediated cell growth signaling pathways through signaling pathways independent of protease inhibitory function. |
| Anti-apoptotic protection | In various cell types such as lymphocytes, it exerts the function of cell survival factors through the CD63/PI3K signaling cascade reaction. |
| Fibrosis promotion | When the inhibitory function is imbalanced, it leads to an imbalance in the MMP/TIMP ratio, promoting excessive collagen deposition and tissue fibrosis. |
This protein exhibits broad-spectrum but differentiated inhibitory activity against various matrix metalloproteinases (such as MMP-1, MMP-2, MMP-3, and MMP-9), with an inhibition constant (Ki) at the nanomolar level. This highly efficient inhibitory property makes it a key regulatory factor for the homeostasis of the tissue microenvironment.
Applications of TIMP1 and TIMP1 Antibody in Literature
1. Qiu, Xiaode, et al. "Unraveling TIMP1: a multifaceted biomarker in colorectal cancer." Frontiers in Genetics 14 (2023): 1265137. https://doi.org/10.3389/fgene.2023.1265137
This study, through bioinformatics and experimental verification, found that TIMP1 is a key biomarker for colorectal cancer. Its high expression is associated with poor prognosis, immune cell infiltration, immune checkpoint expression, and ferroptosis inhibition, and it also affects drug sensitivity.
2. Liu, Hui, et al. "miR-6745-TIMP1 axis inhibits cell growth and metastasis in gastric cancer." Aging (albany NY) 13.21 (2021): 24402. https://doi.org/10.18632/aging.203688
This study found that in the ovaries of goats, TIMP1 is highly expressed in granulosa cells, which can promote cell proliferation and estradiol secretion, and upregulate the expression of lambing related genes such as BMPR-1B, indicating that TIMP1 is a key molecule regulating the reproductive performance of goats.
3. Manríquez-Treviño, Yolanda, et al. "Human TIMP1 Is a Growth Factor That Improves Oocyte Developmental Competence." BioTech 12.4 (2023): 60. https://doi.org/10.3390/biotech12040060
This study found that adding TIMP1 to the in vitro culture of bovine oocytes can effectively maintain the dense structure of the cumulus-oocyte complex, promote oocyte maturation to metaphase II, and improve the distribution of cortical granules, thereby significantly enhancing their subsequent ability to develop into blastocysts.
4. Shou, Yi, et al. "TIMP1 indicates poor prognosis of renal cell carcinoma and accelerates tumorigenesis via EMT signaling pathway." Frontiers in Genetics 13 (2022): 648134. https://doi.org/10.3389/fgene.2022.648134
This study confirmed that TIMP1 is significantly upregulated in renal cell carcinoma and is an independent risk factor for poor prognosis in patients. TIMP1 can promote the proliferation, migration and invasion of cancer cells, and its mechanism of action is related to the activation of the epithelial-mesenchymal transition signaling pathway.
5. Ishihara, Rei, et al. "Myeloma microenvironmental TIMP1 induces the invasive phenotype in fibroblasts to modulate disease progression." International Journal of Molecular Sciences 24.3 (2023): 2216. https://doi.org/10.3390/ijms24032216
This study reveals that the level of TIMP1 derived from multiple myeloma cells increases with disease progression and is associated with a poor prognosis. TIMP1 does not directly act on cancer cells but promotes the invasion and spread of multiple myeloma by enhancing the invasive ability of fibroblasts, thus serving as a potential therapeutic target.
Creative Biolabs: TIMP1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality TIMP1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom TIMP1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our TIMP1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Manríquez-Treviño, Yolanda, et al. "Human TIMP1 Is a Growth Factor That Improves Oocyte Developmental Competence." BioTech 12.4 (2023): 60. https://doi.org/10.3390/biotech12040060
Anti-TIMP1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CASQ1 Recombinant Antibody (CBFYC-0863) (CBMAB-C0918-FY)

-
Mouse Anti-AGO2 Recombinant Antibody (V2-634169) (CBMAB-AP203LY)

-
Mouse Anti-CD247 Recombinant Antibody (6B10.2) (CBMAB-C1583-YY)

-
Mouse Anti-ARID3A Antibody (A4) (CBMAB-0128-YC)

-
Mouse Anti-AHCYL1 Recombinant Antibody (V2-180270) (CBMAB-A1703-YC)

-
Mouse Anti-FN1 Monoclonal Antibody (D6) (CBMAB-1240CQ)

-
Rat Anti-C5AR1 Recombinant Antibody (8D6) (CBMAB-C9139-LY)

-
Rat Anti-ADGRE4 Recombinant Antibody (V2-160163) (CBMAB-F0011-CQ)

-
Mouse Anti-AKR1B1 Antibody (V2-2449) (CBMAB-1001CQ)

-
Mouse Anti-FLI1 Recombinant Antibody (CBXF-0733) (CBMAB-F0435-CQ)

-
Mouse Anti-DDC Recombinant Antibody (8E8) (CBMAB-0992-YC)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Rat Anti-CD34 Recombinant Antibody (MEC 14.7) (CBMAB-C10196-LY)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-ADGRE5 Recombinant Antibody (V2-360335) (CBMAB-C2088-CQ)

-
Mouse Anti-ABL2 Recombinant Antibody (V2-179121) (CBMAB-A0364-YC)

-
Mouse Anti-4-Hydroxynonenal Recombinant Antibody (V2-502280) (CBMAB-C1055-CN)

-
Mouse Anti-CDKL5 Recombinant Antibody (CBFYC-1629) (CBMAB-C1689-FY)

-
Rabbit Anti-CAMK2A Recombinant Antibody (BA0032) (CBMAB-0137CQ)

-
Mouse Anti-AZGP1 Recombinant Antibody (CBWJZ-007) (CBMAB-Z0012-WJ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






