ACP5 Antibodies
Background
ACP5 gene encodes an acid phosphatase mainly expressed in osteoclasts. This protein participates in the regulation of bone resorption microenvironment through the hydrolysis of phosphate ester bonds, and its activity directly affects bone mineral balance. Research has found that defects in ACP5 are closely related to hereditary bone metabolic diseases (such as spastic paraplegia type 35), and its mutations can lead to abnormal bone remodeling and neurological symptoms. This gene was first identified through localization cloning technology in 1996, and its protein crystal structure was resolved in 2009, revealing a unique binuclear iron center catalytic mechanism. As a key component of the bone metabolic pathway, ACP5 has become an important research model for diseases such as osteoporosis and osteosclerosis, providing a molecular basis for the development of therapeutic strategies targeting bone resorption.
Structure of ACP5
The protein encoded by the ACP5 gene (also known as TRAP or Lysosomal Acid Phosphatase) has a molecular weight of approximately 35-40 kDa, and the difference mainly stems from the varying degrees of post-translational glycosylation modification.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | ~37 | ~36 | ~38 |
| Primary Structural Differences | Contains two characteristic acid phosphatase domains | The homology with human is high and the active site is conserved | Sequence is highly similar, is species-specific glycosylation |
This protein is composed of approximately 325 amino acids, and its tertiary structure forms a typical acid phosphatase fold, with a core βαβα bilayer architecture. The active center contains a binuclear iron cluster (Fe3+-Fe3+), which is the direct catalytic site for the hydrolysis of phosphate ester bonds. Two key aspartic acid residues are responsible for coordinating metal ions, while a conserved histidine residue plays a crucial role in substrate binding and catalytic processes.
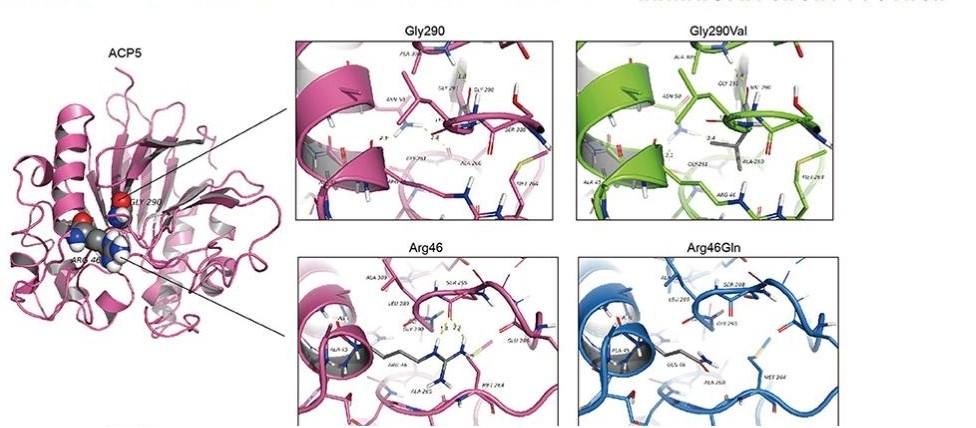 Fig. 1 Structure modeling of wild type and p.Gly290Val mutation of ACP5.1
Fig. 1 Structure modeling of wild type and p.Gly290Val mutation of ACP5.1
Key structural properties of ACP5:
- Typical acid phosphatase folding structure
- The dual-core iron center serves as the core of catalytic activity
- Highly conservative substrate bonding pocket
Functions of ACP5
The core function of the protein encoded by the ACP5 gene (tartrate-resistant acid phosphatase, TRAP) is to participate in the metabolic regulation of bone tissue. However, it also involves a variety of pathophysiological processes, including immune responses and disease progression.
| Function | Description |
| Regulation of bone mineralization | In osteoclasts, phosphate esters are hydrolyzed to destroy the inorganic salt matrix of bone and create an acidic microenvironment for bone resorption. |
| Regulation of immune cells | Expressed in antigen-presenting cells, it participates in the regulation of inflammatory signaling pathways and affects the function of macrophages. |
| Pathological calcification | Abnormal activity is closely related to pathological mineralization processes such as vascular calcification and articular cartilage calcification. |
| Disease biomarkers | The activity level of TRAP in serum is regarded as an important clinical indicator of bone resorption rate and certain bone metabolic diseases. |
| Cancer cell interactions | In the microenvironment of bone metastases, the colonization and growth of cancer cells are affected by regulating the local phosphate concentration. |
The kinetic curve of this enzyme shows the best activity under the acidic pH of the bone resorption microenvironment, which is highly consistent with its physiological role in efficiently degrading bone matrix components during bone resorption.
Applications of ACP5 and ACP5 Antibody in Literature
1. Ramesh, Janani, et al. "Characterisation of ACP5 missense mutations encoding tartrate-resistant acid phosphatase associated with spondyloenchondrodysplasia." PLoS One 15.3 (2020): e0230052. https://doi.org/10.1371/journal.pone.0230052
The article indicates that biallelic mutations in the ACP5 gene lead to SPENCD disease. Research has found that although all mutations can generate TRACP proteins, they all cause the loss of their enzymatic activity and hinder their correct folding and intracellular processing, resulting in blocked protein secretion and impaired function.
2. Liang, Qian, et al. "RANK promotes colorectal cancer migration and invasion by activating the Ca2+-calcineurin/NFATC1-ACP5 axis." Cell death & disease 12.4 (2021): 336. https://doi.org/10.1038/s41419-021-03642-7
Research has found that in colorectal cancer, RANK triggers calcium oscillations by activating the PLCγ-STIM1 pathway, which then upregulates the expression of ACP5 through calcineurin /NFATC1 axis transcription, ultimately driving cancer metastasis.
3. Hong, Soon-Min, et al. "Novel Mutations in ACP5 and SAMHD1 in a patient with pediatric systemic lupus erythematosus." Frontiers in Pediatrics 10 (2022): 885006. https://doi.org/10.3389/fped.2022.885006
In this study, a child with pSLE was reported to carry novel mutations in genes such as ACP5. Analysis shows that ACP5 and its interacting genes are jointly enriched in multiple immune pathways, and their variations may work in synergy with SAMHD1 to disrupt the type I interferon pathway, driving the occurrence of diseases.
4. Zhao, Yu-Ting, et al. "Adaptation of prelimbic cortex mediated by IL-6/STAT3/Acp5 pathway contributes to the comorbidity of neuropathic pain and depression in rats." Journal of Neuroinflammation 19.1 (2022): 144. https://doi.org/10.1186/s12974-022-02503-0
Studies have shown that nerve injury induces the activation of IL-6/STAT3 signals in the anterior limbic cortex, thereby transcriptively upregulating ACP5. The increased expression of ACP5 inhibits the activity of pyramidal neurons and is a key mechanism driving the comorbidity of neuropathic pain and depression.
5. Szaluś-Jordanow, Olga, et al. "A primary multiple pleomorphic rhabdomyosarcoma of the heart in an adult dog." BMC Veterinary Research 19.1 (2023): 137. https://doi.org/10.1186/s10020-024-00856-1
Research has found that the expression of ACP5 is upregulated after myocardial infarction (MI). ACP5 affects the stability of β-catenin by regulating ERK-mediated GSK3β phosphorylation, thereby promoting the proliferation, migration and phenotypic transformation of cardiac fibroblasts and exacerbating cardiac fibrosis.
Creative Biolabs: ACP5 Antibodies for Research
Creative Biolabs specializes in the production of high-quality ACP5 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ACP5 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ACP5 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Hong, Soon-Min, et al. "Novel Mutations in ACP5 and SAMHD1 in a patient with pediatric systemic lupus erythematosus." Frontiers in Pediatrics 10 (2022): 885006. https://doi.org/10.3389/fped.2022.885006
Anti-ACP5 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-APCS Recombinant Antibody (CBYC-A663) (CBMAB-A3054-YC)

-
Mouse Anti-BACE1 Recombinant Antibody (CBLNB-121) (CBMAB-1180-CN)

-
Mouse Anti-ARIH1 Recombinant Antibody (C-7) (CBMAB-A3563-YC)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-BAX Recombinant Antibody (CBYY-0216) (CBMAB-0217-YY)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

-
Mouse Anti-ALDOA Recombinant Antibody (A2) (CBMAB-A2316-YC)

-
Mouse Anti-BZLF1 Recombinant Antibody (BZ.1) (CBMAB-AP705LY)

-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

-
Mouse Anti-4-Hydroxynonenal Recombinant Antibody (V2-502280) (CBMAB-C1055-CN)

-
Mouse Anti-AMACR Recombinant Antibody (CB34A) (CBMAB-CA034LY)

-
Mouse Anti-AP4E1 Recombinant Antibody (32) (CBMAB-A2996-YC)

-
Rabbit Anti-ALK (Phosphorylated Y1278) Recombinant Antibody (D59G10) (PTM-CBMAB-0035YC)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-ADIPOR1 Recombinant Antibody (V2-179982) (CBMAB-A1368-YC)

-
Mouse Anti-BCL6 Recombinant Antibody (CBYY-0435) (CBMAB-0437-YY)

-
Mouse Anti-EPO Recombinant Antibody (CBFYR0196) (CBMAB-R0196-FY)

-
Mouse Anti-ALX1 Recombinant Antibody (96k) (CBMAB-C0616-FY)

-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






