ASNS Antibodies
Background
ASNS are a key metabolic enzyme, mainly found in tissues such as the liver, pancreas and brain of vertebrates. This enzyme catalyzes the synthesis of aspartic acid and glutamine into asparagine, a process that is crucial for maintaining the amino acid balance within cells, supporting protein synthesis and the survival of tumor cells. Studies have shown that the expression level of ASNS is associated with chemotherapy resistance in certain cancers, making it a research target for tumor treatment. This enzyme was first identified in the 1960s. Its structure and functional mechanism were gradually clarified through techniques such as X-ray crystallography, providing an important basis for understanding metabolic regulation, cellular stress response and disease occurrence. In recent years, research on the adaptive regulation of ASNS under nutritional deficiency conditions has further revealed their core role in cell survival.
Structure of ASNS
Myoglobin is a relatively small protein with a molecular weight of approximately 16.7 kDa. This weight may slightly vary between species due to minor differences in amino acid sequence.
| Species | Human | Mice | Rats | Bovine |
| Molecular Weight (kDa) | 64 | 63.5 | 63.8 | 64.2 |
| Primary Structural Differences | Contains catalytic domain and glutamine binding site | Highly conservative, similar to human ASNS | Key catalytic residues | Adapt to different metabolic needs |
ASNS are composed of approximately 561 amino acids, and their tertiary structure contains two main functional domains: the glutaminase domain (responsible for the hydrolysis of glutamine) and the synthase domain (catalyzing the amination of aspartic acid). This enzyme relies on ATP and Mg2+ as cofactors, and its active center maintains catalytic function through a conserved cysteine-histidine-aspartic acid triad. The expression of ASNS is regulated by amino acid starvation response (AAR) and endoplasmic reticulum stress (ERS) pathways, and is particularly upregulated in the tumor microenvironment, which is closely related to the drug resistance of cancer cells.
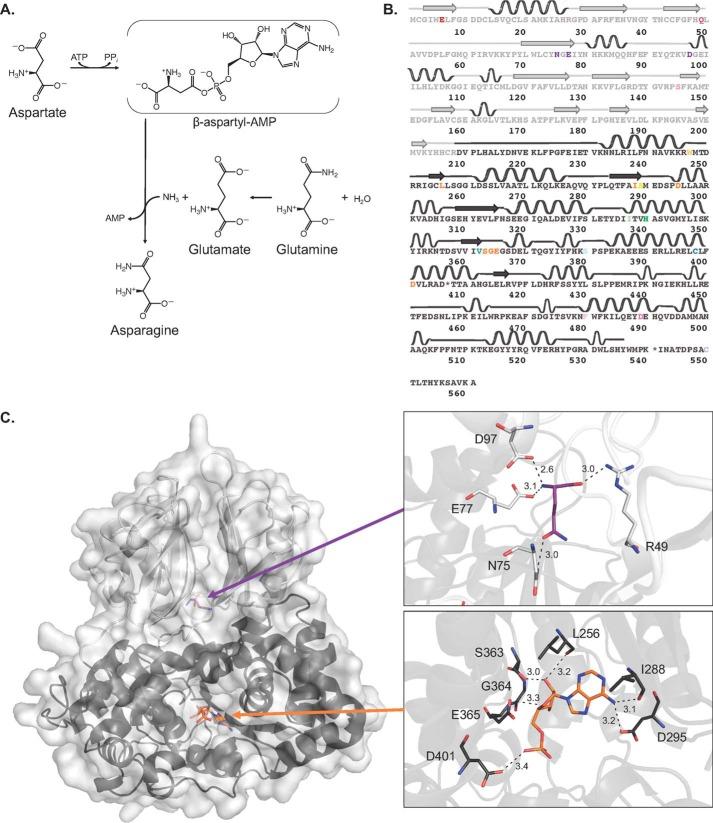 Fig. 1 Mechanism and structural features of human asparagine synthetase.1
Fig. 1 Mechanism and structural features of human asparagine synthetase.1
Functions of ASNS
The main function of ASNS is to catalyze the synthesis of asparagine and maintain the amino acid balance within cells, but it also plays a key role in various physiological and pathological processes.
| Function | Description |
| Asparagine synthesis | Catalyzing aspartate and glutamine to asparagine, supporting protein synthesis and cell proliferation. |
| Tumor metabolic adaptation | Under conditions of nutritional deficiency (such as low glutamine), the upregulation of ASNS helps cancer cells survive and promotes chemotherapy resistance. |
| Endoplasmic reticulum stress response | By regulating the ATF4-CHOP pathway, it alleviates endoplasmic reticulum stress and maintains cellular homeostasis. |
| Nervous system protection | Maintaining asparagine levels in the brain affects neurotransmitter synthesis and neuronal function. |
| Immune regulation | Influence T cell metabolism and function, to participate in the immune response regulation. |
The activity of ASNS is dynamically regulated by the mTORC1 signaling pathway and amino acid starvation. Its expression level is related to the prognosis of various cancers (such as leukemia and pancreatic cancer), making it a potential therapeutic target.
Applications of ASNS and ASNS Antibody in Literature
1. Chen, Jiawei, et al. "ATF4 inhibits tumor development and mediates p-GCN2/ASNS upregulation in colon cancer." Scientific Reports 14.1 (2024): 13042.https://doi.org/10.1038/s41598-024-63895-y
This study explored the mechanism by which ATF4 inhibits colon cancer by regulating the expression of p-GCN2 and ASNS. Experiments have found that ATF4 can bind to the ASNS promoter region, up-regulate the protein expression detected by ASNS antibodies, thereby inhibiting the proliferation and migration of tumor cells and promoting apoptosis. ASNS may become a new target for the treatment of colon cancer.
2. Noree, Chalongrat, Elena Monfort, and Vorasuk Shotelersuk. "Human asparagine synthetase associates with the mitotic spindle." Biology Open 7.12 (2018): bio038307. https://doi.org/10.1242/bio.038307
Research has found that human asparagine synthase (ASNS) not only participates in metabolic reprogramming but also plays a potential role in mitosis. ASNS antibody detection shows that it is located in the centrosome and spindle, and this localization is regulated by nocodaazole and asparaginase, suggesting that ASNS may have a "part-time function" of promoting cancer and providing a new target for cancer treatment.
3. Sydow, Jasmin F., et al. "Structure-based prediction of asparagine and aspartate degradation sites in antibody variable regions." PloS one 9.6 (2014): e100736. https://doi.org/10.1371/journal.pone.0100736
This study, through mass spectrometry analysis, found that the degradation hotspots of asparagine (Asn) and aspartic acid (Asp) in the variable region of antibodies are related to their conformational flexibility and the size of the C-terminal side chain. A precise prediction model for the degradation tendency of the CDR region was established, providing a new strategy for the stability optimization of ASNS antibody drugs.
4. Grima-Reyes, Manuel, et al. "Tumoral microenvironment prevents de novo asparagine biosynthesis in B cell lymphoma, regardless of ASNS expression." Science advances 8.27 (2022): eabn6491. https://www.science.org/doi/full/10.1126/sciadv.abn6491
This study revealed through isotope tracer technology that regardless of the baseline expression level of ASNS antibody detection, asparagine synthase (ASNS) alone could not predict the sensitivity of cancer cells to ASNase treatment. Importantly, the glutaminase activity of ASNase plays a key role in its anti-cancer effect, which provides significant implications for clinical treatment strategies.
5. Lomelino, Carrie L., et al. "Asparagine synthetase: Function, structure, and role in disease." Journal of Biological Chemistry 292.49 (2017): 19952-19958. https://doi.org/10.1074/jbc.R117.819060
Studies have shown that asparagine synthase (ASNS) plays a key role in cellular stress responses, and its expression level is negatively correlated with the therapeutic effect of asparagine enzyme. Research has found that ASNS gene mutations can lead to developmental defects. ASNS antibody detection shows that most of the mutation sites are located in the catalytic active region, which provides a molecular basis for the diagnosis and treatment of related diseases.
Creative Biolabs: ASNS Antibodies for Research
Creative Biolabs specializes in the production of high-quality ASNS antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ASNS Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ASNS antibodies, custom preparations, or technical support, contact us at email.
Reference
- Lomelino, Carrie L., et al. "Asparagine synthetase: Function, structure, and role in disease." Journal of Biological Chemistry 292.49 (2017): 19952-19958. https://doi.org/10.1074/jbc.R117.819060
Anti-ASNS antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-C5AR1 Recombinant Antibody (R63) (CBMAB-C9553-LY)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

-
Rabbit Anti-BRCA2 Recombinant Antibody (D9S6V) (CBMAB-CP0017-LY)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Rabbit Anti-CAMK2A Recombinant Antibody (BA0032) (CBMAB-0137CQ)

-
Mouse Anti-CA9 Recombinant Antibody (CBXC-2079) (CBMAB-C0131-CQ)

-
Mouse Anti-APC Recombinant Antibody (CBYC-A661) (CBMAB-A3036-YC)

-
Mouse Anti-BrdU Recombinant Antibody (IIB5) (CBMAB-1038CQ)

-
Mouse Anti-CECR2 Recombinant Antibody (CBWJC-2465) (CBMAB-C3533WJ)

-
Mouse Anti-AMOT Recombinant Antibody (CBYC-A564) (CBMAB-A2552-YC)

-
Mouse Anti-FOXL1 Recombinant Antibody (CBXF-0845) (CBMAB-F0462-CQ)

-
Mouse Anti-DES Monoclonal Antibody (440) (CBMAB-AP1857LY)

-
Mouse Anti-EPO Recombinant Antibody (CBFYR0196) (CBMAB-R0196-FY)

-
Rabbit Anti-BAD (Phospho-Ser136) Recombinant Antibody (CAP219) (CBMAB-AP536LY)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






