ATRX Antibodies
Background
ATRX gene encodes a chromatin remodeling protein, which is widely expressed in various tissues of vertebrates, especially in the central nervous system. This protein directly affects chromatin structure and gene expression by regulating telomere maintenance and histone modification and plays a key role in neural development and cell differentiation. Research has found that ATRX mutations are closely related to various X-linked intellectual disability syndromes, and patients often present with hematopoietic abnormalities such as α -thalassemia. This gene was first identified through linkage analysis in 1990, and the analysis of its protein structure revealed the characteristic conformation of the ATpase domain of the SWI/SNF family. Continuous research on ATRX not only deepens the understanding of epigenetic regulatory mechanisms but also provides an important molecular basis for the diagnosis and treatment of chromatin remodeling anomaly-related diseases.
Structure of ATRX
The ATRX protein is a large chromatin remodeling protein with a molecular weight of approximately 280 kDa. This protein belongs to the SWI/SNF2 protein family. Its size is highly conserved among different species, and the main differences are reflected in non-core functional regions.
| Species | Human | Mouse | Zebrafish | Fruit fly |
| Molecular Weight (kDa) | ~280 | ~278 | ~275 | ~250 |
| Primary Structural Differences | Containing AT-hook and ADD domains, it regulates gene expression and telomere maintenance | Function similar to human height, is a common disease model | Play a key role in early development | Homologous gene for XNP, function relatively simplified |
This protein contains a characteristic helicase domain, whose core function depends on the energy generated by ATP hydrolysis to alter chromatin structure. The ADD domain is responsible for identifying specific modification states of histone H3, which is key to its targeting of specific genomic regions. The helicase domain exerts ATP-dependent nucleic acid translocation activity, causing nucleosome sliding, and thus plays a core role in epigenetic regulation, the transcriptional maintenance of ribosomal DNA repeat sequences, and the protection of telomere integrity.
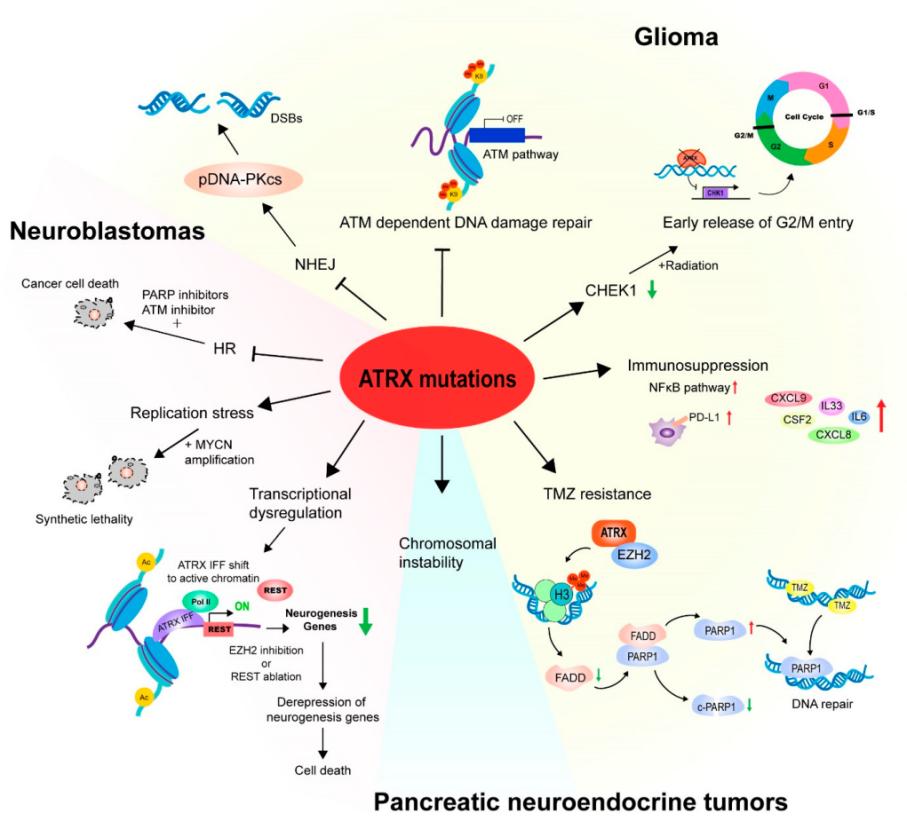 Fig. 1 ATRX mutations and cancers.1
Fig. 1 ATRX mutations and cancers.1
Key structural properties of ATRX:
- Characteristic helicase/ATPase domain
- Recognition of the histone modified ADD zinc finger domain
- AT-hook motif combined with DNA
Functions of ATRX
The core function of the ATRX gene is to act as a chromatin remodeling factor. In addition, it also plays a key role in maintaining genomic stability.
| Function | Description |
| Chromatin remodeling | The utilization of ATP hydrolysis energy to alter the position of nucleosomes and regulate the transcriptional activity of genes is crucial for brain development. |
| Epigenetic regulation | Structural domain specificity identification through its ADD histone H3K9me3 modifications, recruited to the heterochromatin regions such as telomeres. |
| Telomere maintenance | ATRX loss is a key molecular event that activates the alternative telomere elongation pathway in ALT-positive tumors. |
| Genomic stability | By maintaining the heterochromatin structure in repetitive sequence regions such as telomeres and centromeres, chromosomal abnormalities can be prevented. |
| Cell fate determination | Its regulatory function at the chromatin level directly affects the proliferation and differentiation balance of neural precursor cells. |
Unlike typical SWI/SNF complexes that specifically act on euchromatin, ATRX is mainly enriched in heterochromatin regions. This targeting specificity makes its function more specific and plays a key role in neurodevelopment and tumor suppression. Its mutations are closely related to various diseases such as X-linked intellectual disability syndrome and pancreatic neuroendocrine tumors.
Applications of ATRX and ATRX Antibody in Literature
1. Pang, Ying, et al. "The chromatin remodeler ATRX: role and mechanism in biology and cancer." Cancers 15.8 (2023): 2228. https://doi.org/10.3390/cancers15082228
The article indicates that ATRX is an important chromatin remodeling protein, and its mutations are closely related to the occurrence and development of various cancers. It maintains genomic stability by regulating mechanisms such as histone H3.3 deposition and DNA damage repair, and has significant clinical significance in tumors such as glioma. In-depth research on the function of ATRX will provide a new direction for targeted therapy.
2. Clatterbuck Soper, Sarah F., and Paul S. Meltzer. "ATRX/DAXX: Guarding the Genome against the Hazards of ALT." Genes 14.4 (2023): 790. https://doi.org/10.3390/genes14040790
The article indicates that the ATRX/DAXX complex maintains telomere heterochromatin stability through functions such as depositing histone H3.3. The inactivation of this complex triggers a homologous recombination mechanism called ALT, thereby maintaining the telomere length of cancer cells. This reveals the crucial role of ATRX deletion in cancer development.
3. Yuan, Kejia, et al. "Mutant ATRX: pathogenesis of ATRX syndrome and cancer." Frontiers in Molecular Biosciences 11 (2024): 1434398. https://doi.org/10.3389/fmolb.2024.1434398
The article indicates that ATRX is a key transcriptional regulatory factor, and its mutation can lead to ATRX syndrome, which is commonly seen in various cancers such as glioma. These mutations can disrupt chromosomal stability and interactions with key proteins such as DAXX, eventually leading to a variety of diseases.
4. Valenzuela, Martina, et al. "The multiple facets of ATRX protein." Cancers 13.9 (2021): 2211. https://doi.org/10.3390/cancers13092211
The article indicates that ATRX is an important epigenetic regulatory factor and tumor suppressor gene, and its mutations are widely present in various cancers. As the "guardian" of the genome, it plays a core role in maintaining heterochromatin, telomere function and genomic stability, and its dysfunction can directly lead to tumorigenesis.
5. Nandakumar, Pravanya, Alireza Mansouri, and Sunit Das. "The role of ATRX in glioma biology." Frontiers in oncology 7 (2017): 236. https://doi.org/10.3389/fonc.2017.00236
The article indicates that ATRX is a key biomarker for the molecular typing of central nervous system tumors by the WHO, and its status is crucial for the classification of diffuse gliomas. This review will explore the role of ATRX in normal cell biology, its functions in the occurrence and development of glioma and epigenetic regulation, and look forward to the challenges and prospects of its clinical application.
Company A: ATRX Antibodies for Research
Company A specializes in the production of high-quality ATRX antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ATRX Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ATRX antibodies, custom preparations, or technical support, contact us at email.
Reference
- Pang, Ying, et al. "The chromatin remodeler ATRX: role and mechanism in biology and cancer." Cancers 15.8 (2023): 2228. https://doi.org/10.3390/cancers15082228
Anti-ATRX antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-ARID3A Antibody (A4) (CBMAB-0128-YC)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-ATP1B1 Recombinant Antibody (E4) (CBMAB-0463-LY)

-
Mouse Anti-ACLY Recombinant Antibody (V2-179314) (CBMAB-A0610-YC)

-
Human Anti-SARS-CoV-2 S1 Monoclonal Antibody (CBFYR-0120) (CBMAB-R0120-FY)

-
Mouse Anti-CHRNA9 Recombinant Antibody (8E4) (CBMAB-C9161-LY)

-
Mouse Anti-FOSB Recombinant Antibody (CBXF-3593) (CBMAB-F2522-CQ)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Mouse Anti-ATP1A2 Recombinant Antibody (M7-PB-E9) (CBMAB-A4013-YC)

-
Mouse Anti-CALR Recombinant Antibody (CBFYC-0763) (CBMAB-C0818-FY)

-
Mouse Anti-COL1A2 Recombinant Antibody (CF108) (V2LY-1206-LY626)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Mouse Anti-HTLV-1 gp46 Recombinant Antibody (CBMW-H1006) (CBMAB-V208-1154-FY)

-
Mouse Anti-COL12A1 Recombinant Antibody (CBYY-C3117) (CBMAB-C4560-YY)

-
Mouse Anti-4-Hydroxynonenal Recombinant Antibody (V2-502280) (CBMAB-C1055-CN)

-
Mouse Anti-CORO1A Recombinant Antibody (4G10) (V2LY-1206-LY806)

-
Mouse Anti-ARSA Recombinant Antibody (CBYC-A799) (CBMAB-A3679-YC)

-
Mouse Anti-BIRC3 Recombinant Antibody (16E63) (CBMAB-C3367-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






