CD22 Antibodies
Background
CD22 is a type I transmembrane lectin protein mainly expressed on the surface of B lymphocytes. As a key negative regulatory factor of B cell receptors (BCRS), it inhibits the excessive activation of B cells by recruiting phosphatases through the intracellular immune receptor tyrosine inhibitory motif (ITIM). This gene is located in the q13.2 region of human chromosome 19. The protein it encodes participates in the maintenance of B cell tolerance and the regulation of immune responses by specifically recognizing the sialic acid glycosylation modification linked by α2,6-Since its discovery in the 1980s, CD22 has become an important target for the research of autoimmune diseases and B-cell malignancies. Antibody-drug conjuges developed against it, such as Inotuzumab Ozogamicin, have been applied in the clinical treatment of acute lymphoblastic leukemia. Research on the structure and function of CD22 has greatly promoted the understanding of immune regulatory mechanisms and the development of targeted therapies.
Structure of CD22
CD22 is a type I transmembrane glycoprotein with a molecular weight of approximately 95-140 kDa. The difference in molecular weight mainly results from the varying degrees of glycosylation modification.
| Species | Human | Mouse | Rhesus monkey | Rat |
| Molecular Weight (kDa) | 130-140 | 95-105 | 125-135 | 100-110 |
| Primary Structural Differences | Sample contains seven Ig domain structure | CD22 67% homology with people | CD22 is highly conservative with humans | The intracellular segment ITIM motif is conserved |
This protein is composed of 807 amino acids, and its extracellular segment contains 7 immunoglobulin-like domains (among which the N-terminal V-type domain is responsible for binding to α2, 6-sialic acid). The third immunoglobulin domain of CD32 mediates protein dimerization, while the intracellular segment contains three classical immune receptor tyrosine inhibitory motifs (ITIMs), which can recruit SHP-1 phosphatase after phosphorylation. Its ligand binding characteristics depend on sialidase activity, and this unique recognition mechanism enables it to play a key regulatory role in B-cell signal transduction.
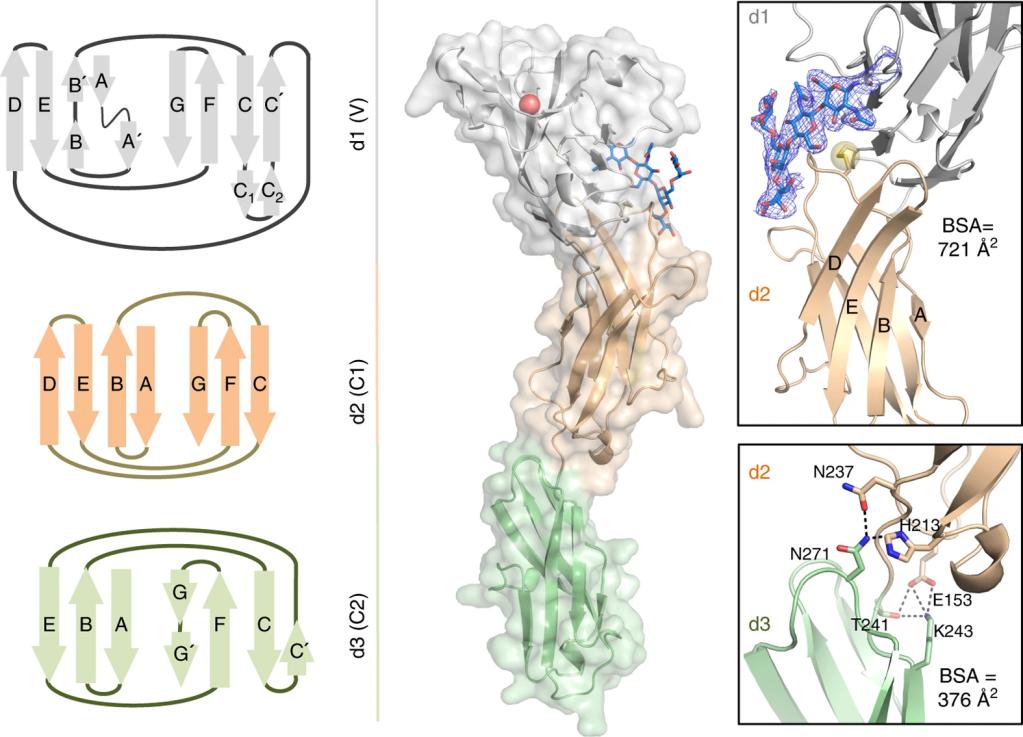 Fig. 1 Three-dimensional structure of human CD22.1
Fig. 1 Three-dimensional structure of human CD22.1
Key structural properties of CD22:
- The extracellular segment contains seven immunoglobulin-like domains (Ig1-Ig7)
- The V-type Ig domain specifically recognizes the α2, 6-linked sialic acid glycosylated ligand
- The transmembrane region forms a co-receptor structure with the BCR complex
- Intracellular section contains three conservative motif to suppress the immune receptor tyrosine kinases (ITIM)
- The 5th and 6Ig domains mediate cis-interactions with CD45RO
Functions of CD22
The main function of CD22 is to act as a negative regulatory factor for B-cell immune responses. However, it is also involved in a variety of immune regulatory processes, including intercellular interactions and signal transduction regulation.
| Function | Description |
| Inhibition of B cell activation | Recruit SHP-1 phosphatase through the ITIM motif to inhibit the excessive activation of the BCR signaling pathway. |
| Maintenance of immune tolerance | Recognize the autoantigens of sialacidification and maintain B-cell autoimmune tolerance. |
| Regulation of cell adhesion | The trans-interaction with CD22 ligands mediated by the V-set domain regulates the contact between B cells and other immune cells. |
| Signal transduction modulation | Interact with the CD19/CD21 complex to modulate the activation threshold of B cells. |
| Regulation of antigen presentation | Affect the B cell antigen presented efficiency, indirect regulating T cell response. |
The binding characteristics of CD22 to ligands are characterized by calcium ion-dependent low-affinity interactions (Kd≈200μM). This rapid dissociation kinetics enables it to dynamically scan the glycoylation patterns on the cell surface, thereby regulating the functional status of B cells in real time.
Applications of CD22 and CD22 Antibody in Literature
1. Okuzono, Yuumi, et al. "B-cell immune dysregulation with low soluble CD22 levels in refractory seronegative myasthenia gravis." Frontiers in Immunology 15 (2024): 1382320. https://doi.org/10.3389/fimmu.2024.1382320
This study, through single-cell sequencing and plasma proteomics analysis, found that patients with refractory seronegative myasthenia gravis (MG) had abnormal proliferation of B cells and a reduction in regulatory T cells. The level of soluble CD22 derived from the mature line of B cells in their plasma was significantly decreased and was related to the severity of the disease, suggesting that targeting B cells and CD22 may become a new treatment strategy.
2. Dai, Hanren, et al. "Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia." Journal of hematology & oncology 13.1 (2020): 30. https://doi.org/10.1186/s13045-020-00856-8
This study conducted a Phase I trial of CD19/CD22 bispecific CAR-T treatment for relapsed/refractory B-ALL. The results showed that this therapy could effectively eliminate cancer cells. All six patients achieved MRD-negative complete remission, and no neurotoxicity occurred. However, one patient relapsed accompanied by CD19 deletion and down-regulation of CD22 expression, suggesting that the issue of antigen escape needs further attention.
3. Cordoba, Shaun, et al. "CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial." Nature medicine 27.10 (2021): 1797-1805. https://doi.org/10.1038/s41591-021-01497-1
This study conducts a Phase I clinical trial of bispecific CAR-T (AUTO3) targeting CD19 and CD22 in children and young patients with relapsed/refractory B-ALL. The results showed that the treatment was safe, with no serious toxic or side effects, and the complete remission rate reached 86% at one month. However, the long-term insufficient persistence of CAR-T cells leads to recurrence, suggesting that the maintenance strategy needs to be improved to exert the therapeutic potential of dual-target therapy.
4. Fergusson, Nathan J., et al. "A systematic review and meta-analysis of CD22 CAR T-cells alone or in combination with CD19 CAR T-cells." Frontiers in Immunology 14 (2023): 1178403. https://doi.org/10.3389/fimmu.2023.1178403
This review analysis shows that CD22 and CD19/CD22 dual-target CAR-T therapies have high response rates in both acute lymphoblastic leukemia (ALL) and lymphoma (NHL), reaching 68% and 90% (ALL) and 64% and 47% (NHL), respectively. Moreover, the incidence of severe toxic and side effects is low, and the safety is good.
5. Spiegel, Jay Y., et al. "CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial." Nature medicine 27.8 (2021): 1419-1431. https://doi.org/10.1038/s41591-021-01436-0
The article indicates that in response to the issue of easy recurrence after CD19-targeted CAR-T treatment, a Phase I clinical trial for relapsed/refractory B-cell malignant tumors shows that the bispecific CD19-22 CAR-T therapy has good safety and can partially overcome the drug resistance caused by CD19-negative/low expression. However, the targeting efficiency of CD22 and the secretion levels of cytokines still need to be optimized.
Creative Biolabs: CD22 Antibodies for Research
Creative Biolabs specializes in the production of high-quality CD22 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom CD22 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our CD22 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Ereño-Orbea, June, et al. "Molecular basis of human CD22 function and therapeutic targeting." Nature communications 8.1 (2017): 764. https://doi.org/10.1038/s41467-017-00836-6
Anti-CD22 antibodies
 Loading...
Loading...
Hot products 
-
Rat Anti-ABCC11 Recombinant Antibody (V2-179001) (CBMAB-A0236-YC)

-
Rabbit Anti-Acetyl-Histone H3 (Lys36) Recombinant Antibody (V2-623395) (CBMAB-CP0994-LY)

-
Mouse Anti-BBS2 Recombinant Antibody (CBYY-0253) (CBMAB-0254-YY)

-
Mouse Anti-CAPZB Recombinant Antibody (CBYY-C0944) (CBMAB-C2381-YY)

-
Mouse Anti-BLNK Recombinant Antibody (CBYY-0623) (CBMAB-0626-YY)

-
Mouse Anti-BCL2L1 Recombinant Antibody (H5) (CBMAB-1025CQ)

-
Mouse Anti-AAV8 Recombinant Antibody (V2-634028) (CBMAB-AP022LY)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

-
Rabbit Anti-ALDOA Recombinant Antibody (D73H4) (CBMAB-A2314-YC)

-
Mouse Anti-FN1 Monoclonal Antibody (71) (CBMAB-1241CQ)

-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Mouse Anti-C5b-9 Recombinant Antibody (aE11) (CBMAB-AO138LY)

-
Mouse Anti-CFL1 Recombinant Antibody (CBFYC-1771) (CBMAB-C1833-FY)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Mouse Anti-C1QC Recombinant Antibody (CBFYC-0600) (CBMAB-C0654-FY)

-
Mouse Anti-FN1 Monoclonal Antibody (D6) (CBMAB-1240CQ)

-
Mouse Anti-AOC3 Recombinant Antibody (CBYY-0014) (CBMAB-0014-YY)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








