CSAD Antibodies
Background
The CSAD gene encodes cysteine sulfonic acid decarboxylase, which is mainly distributed in the cytoplasm of mammalian liver cells and serves as a key enzyme in the bile acid synthesis pathway, responsible for converting cysteine derivatives into taurine precursors. Its catalytic products are involved in regulating physiological processes such as bile salt binding, central nervous system development and calcium signal transduction. This gene was first identified through cDNA library screening in 1998. The protein it encodes adopts a typical pyridoxal phosphate dependent folding structure, making it an important model for studying the catalytic mechanism of vitamin B6-dependent enzymes. An in-depth analysis of the regulation of CSAD gene expression not only reveals the molecular basis of taurine biosynthesis but also provides a new perspective for the study of pathological mechanisms in metabolic diseases and abnormal liver and gallbladder functions.
Structure of CSAD
The molecular weight of cysteine sulfonic acid decarboxylase encoded by the CSAD gene is approximately 53 kDa. Its precise molecular weight varies slightly among different species, mainly due to specie-specific variations in amino acid sequences.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 53.2 | 52.8 | 53.1 | 53.0 |
| Primary Structural Differences | With C terminal phosphorylation site | The catalytic domain is highly conserved | There are specific mutations in the substrate binding region | 92% homology with human sequence |
This enzyme is composed of approximately 480 amino acid residues and adopts a typical pyridoxal phosphate (PLP) -dependent enzyme folding pattern. Its tertiary structure forms a tight α/β folded barrel, with a PLP cofactor binding cavity at the center, covalently linked to Lys247 through a Schiff base. The active site is composed of a charge relay system formed by the arginine triad (Arg71, Arg97, Arg413), which is responsible for stabilizing the carboxyl group of the substrate. The side chains of Thr322 and Ser156 form a hydrogen bond network, precisely regulating the substrate orientation and catalytic process.
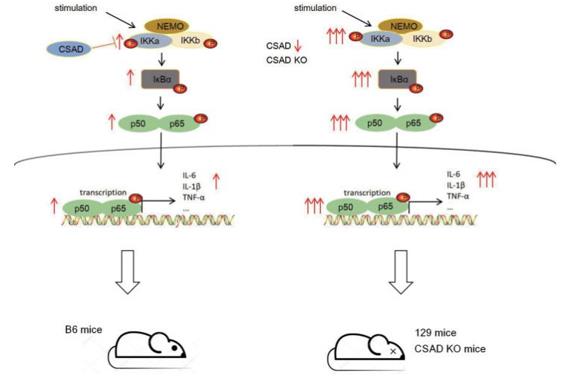 Fig. 1 The mechanisms of CSAD regulate the strength of the NF-κB signaling pathway.1
Fig. 1 The mechanisms of CSAD regulate the strength of the NF-κB signaling pathway.1
Key structural properties of CSAD:
- Typical pyridoxal phosphate (PLP) -dependent enzyme folding architecture
- The conservative active pocket is composed of arginine triplets
- PLP cofactors are covalently linked through Schiff's base
- Substrate channel gating mechanisms ensure catalytic specificity
Functions of CSAD
The core function of the cysteine sulfonic acid decarboxylase encoded by the CSAD gene is a key step in catalyzing the biosynthesis of taurine, and it is also involved in multiple physiological regulatory networks.
| Function | Description |
| Taurine synthesis | Catalyze the decarboxylation of cysteine sulfonic acid to form taurine, which is the rate-limiting step in the biosynthesis of taurine in vivo. |
| Bile acid binding | Its products combine with bile acids to form taurocholic acid, promoting lipid emulsification and absorption. |
| Neural regulation | By maintaining the taurine level in the central nervous system, it regulates GABAergic signaling and calcium homeostasis. |
| Antioxidant defense | Taurine precursors are involved in eliminating reactive oxygen species and protecting liver cells from oxidative damage. |
| Metabolic integration | As a hub for sulfur amino acid metabolism, it connects cysteine metabolism with the bile acid synthesis pathway. |
The reaction catalyzed by this enzyme follows the ping-pong double displacement mechanism, with a Mie constant (Km value) of 0.8mM and an optimal pH of 7.4. These kinetic characteristics ensure that it can efficiently catalyze substrate transformation in the cytoplasm of hepatocytes. Enzyme activity is inhibited by product feedback and regulated by phosphorylation. This precise regulatory mechanism maintains the dynamic balance of taurine metabolism in the body.
Applications of CSAD and CSAD Antibody in Literature
1. Tan, Rongrong, et al. "CSAD ameliorates lipid accumulation in high-fat diet-fed mice." International Journal of Molecular Sciences 23.24 (2022): 15931.https://doi.org/10.3390/ijms232415931
This study, through the analysis of the GEO database and animal models, found that the expression of CSAD was significantly reduced in non-alcoholic fatty liver disease. Overexpression of CSAD in the liver can alleviate the pathological changes of fatty liver. The mechanism may be related to promoting β -oxidation of fatty acids and improving mitochondrial damage, and it is not dependent on the taurine pathway. Studies have shown that CSAD is a potential therapeutic target for NAFLD.
2. Zhang, Yufan, et al. "CSAD inhibits excessive inflammation during viral infections through the NF-κB signaling pathway." Journal of Virology (2025): e00706-25. https://doi.org/10.1128/jvi.00706-25
Research has found that CSAD is a key negative regulatory factor of the innate immune response. It prevents harmful excessive inflammation after viral infection by directly binding to IKKα and inhibiting the excessive activation of the NF-κB signaling pathway. This explains the differences in the intensity of immune responses among different individuals in terms of mechanisms.
3. Wang, Zengbin, et al. "RBM17 promotes hepatocellular carcinoma progression by regulating lipid metabolism and immune microenvironment: implications for therapeutic targeting." Cell Death Discovery 11.1 (2025): 338. https://doi.org/10.1038/s41420-025-02642-2
This study reveals that RBM17, which is highly expressed in liver cancer, promotes exon skipping of the CSAD gene by regulating alternative splicing, thereby facilitating the production of taurocholic acid (T-CA) and the infiltration of M2-type macrophages, and accelerating tumor progression. This indicates that targeting the RBM17-CSAD axis is a potential therapeutic strategy.
4. Abbasian, Nima, et al. "Hepatic cysteine sulphinic acid decarboxylase depletion and defective taurine metabolism in a rat partial nephrectomy model of chronic kidney disease." BMC nephrology 22.1 (2021): 250. https://doi.org/10.1186/s12882-021-02442-7
This study found that in a rat model of chronic kidney disease (CKD), although the mRNA levels of related genes did not change significantly, the expression of the key taurine synthase CSAD protein in the liver was significantly decreased, leading to impaired systemic taurine biosynthesis. Supplementation with L-glutamine failed to improve this condition. This indicates that the loss of CSAD protein is a key link in the reduction of taurine synthesis in CKD.
5. Ma, Hui-fen, et al. "Metabolomic profiling of brain protective effect of edaravone on cerebral ischemia-reperfusion injury in mice." Frontiers in pharmacology 13 (2022): 814942. https://doi.org/10.3389/fphar.2022.814942
This study reveals that edaravone promotes taurine production in the brain by up-regulating CSAD, a key rate-limiting enzyme in taurine synthesis, and thereby inhibits apoptosis of brain endothelial cells, exerting a therapeutic effect on ischemic stroke. CSAD has been confirmed as the target of edalafoxin.
Creative Biolabs: CSAD Antibodies for Research
Creative Biolabs specializes in the production of high-quality CSAD antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom CSAD Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our CSAD antibodies, custom preparations, or technical support, contact us at email.
Reference
- Zhang, Yufan, et al. "CSAD inhibits excessive inflammation during viral infections through the NF-κB signaling pathway." Journal of Virology (2025): e00706-25. https://doi.org/10.1128/jvi.00706-25
Anti-CSAD antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CD24 Recombinant Antibody (HIS50) (CBMAB-C10123-LY)

-
Mouse Anti-ACO2 Recombinant Antibody (V2-179329) (CBMAB-A0627-YC)

-
Mouse Anti-ADAM12 Recombinant Antibody (V2-179752) (CBMAB-A1114-YC)

-
Mouse Anti-ALB Recombinant Antibody (V2-180650) (CBMAB-A2186-YC)

-
Mouse Anti-dsRNA Recombinant Antibody (2) (CBMAB-D1807-YC)

-
Mouse Anti-EMP3 Recombinant Antibody (CBFYE-0100) (CBMAB-E0207-FY)

-
Mouse Anti-ADV Recombinant Antibody (V2-503423) (CBMAB-V208-1364-FY)

-
Mouse Anti-DLL4 Recombinant Antibody (D1090) (CBMAB-D1090-YC)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503416) (CBMAB-V208-1402-FY)

-
Mouse Anti-ALPL Antibody (B4-78) (CBMAB-1009CQ)

-
Mouse Anti-CGAS Recombinant Antibody (CBFYM-0995) (CBMAB-M1146-FY)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Mouse Anti-BIRC3 Recombinant Antibody (315304) (CBMAB-1214-CN)

-
Mouse Anti-ACE2 Recombinant Antibody (V2-179293) (CBMAB-A0566-YC)

-
Rabbit Anti-CCL5 Recombinant Antibody (R0437) (CBMAB-R0437-CN)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-AKR1B1 Antibody (V2-2449) (CBMAB-1001CQ)

-
Mouse Anti-BLNK Recombinant Antibody (CBYY-0623) (CBMAB-0626-YY)

-
Mouse Anti-ALB Recombinant Antibody (V2-363290) (CBMAB-S0173-CQ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






