FADD Antibodies
Background
FADD is a key adaptor protein, mainly present in the cytoplasm of animal cells. This protein binds to death receptors through its death domain, participates in mediating the activation of exogenous apoptotic signaling pathways, and also plays a role in cell cycle regulation and inflammatory responses. In 1995, multiple research teams independently discovered FADD, and its mediated oligomerization mechanism in the death domain provided an important model for apoptosis research. The highly conserved dual-domain architecture of this protein, though simple, is functionally precise. The structural analysis of its interaction network has greatly advanced the research on the molecular mechanisms of programmed cell death, immune regulation, and related disease treatment strategies.
Structure of FADD
Myoglobin is a relatively small protein with a molecular weight of approximately 16.7 kDa. This weight may slightly vary between species due to minor differences in amino acid sequence.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 23.0 | 23.1 | 23.2 | 22.9 |
| Primary Structural Differences | Contains an N-terminal DED domain and a C-terminal DD domain | DED sequence is highly conserved structure domain | DD structure domain and human homology of 95% | Dual domain architecture is basically the same as human architecture |
This protein is composed of 208 amino acids and forms a bifunctional module through the death effector subdomain (DED) at the N-terminal and the death domain (DD) at the C-terminal. The DED domain is mainly responsible for binding to procaspase-8 to form a death-inducing signal complex, while the DD domain directly interacts with death receptors such as Fas/CD95. The two domains are connected by α -helical beams, forming a molecular bridge with signal transduction function. The stability of its tertiary structure plays a decisive role in the precise conduction of apoptotic signals.
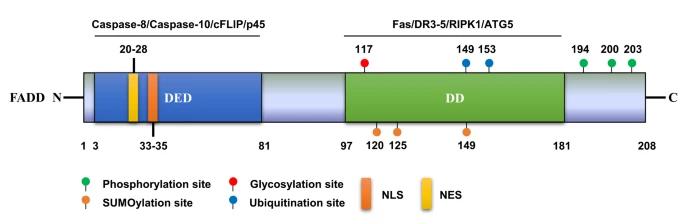 Fig. 1 Protein structure and PTM sites of human FADD.1
Fig. 1 Protein structure and PTM sites of human FADD.1
Key structural properties of FADD:
- Dual-domain architecture (N-end DED and C-end DD)
- Signal transduction hubs are formed between domains through flexible connection zones
- Death domains mediate Fas/TNFR1 superfamily receptor interactions
- Death effect substructure domain responsible for caspase - 8 raise and activation
- Phosphorylation sites (Ser194/Ser203) regulate the efficiency of apoptotic signal transduction
Functions of FADD
The core function of FADD is to mediate exogenous apoptotic signal transduction and simultaneously participate in the regulation of various cellular processes.
| Function | Description |
| Death receptor signal transduction | By binding to the Fas/TNFR1 family receptors through the death domain, the formation of the death-inducing signal complex is initiated. |
| Caspase activates the cascade | procaspase-8 was recruited by means of the death effect subdomain to promote its self-splicing activation. |
| Cell cycle regulation | By affecting the G1/S phase transition through phosphorylation status, deletion results in cell cycle arrest. |
| Regulation of inflammatory response | Interact with TLR signaling pathways that regulate the activation of the nf-kappa B level. |
| Embryonic development participation | In the process of development and T cell selection of the lymphatic system play an important role. |
The activity of FADD is strictly regulated by multi-site phosphorylation, and the intensity of its signal output depends on the conformational changes of the protein and the oligomerization state. This characteristic makes it a key molecular switch in determining cell fate.
Applications of FADD and FADD Antibody in Literature
1. Liu, Ying, et al. "FADD as a key molecular player in cancer progression." Molecular Medicine 28.1 (2022): 132. https://doi.org/10.1186/s10020-022-00560-y
The article indicates that FADD is a key adaptor protein for death receptor-mediated apoptosis and is also involved in various cellular processes such as proliferation and autophagy. Its expression and activity are regulated by mechanisms such as methylation and non-coding RNA, and are closely related to the progression of various cancers. This article reviews the function, regulatory mechanism of FADD and its potential as a cancer biomarker and therapeutic target.
2. Munawar, Umair, et al. "Impaired FADD/BID signaling mediates cross-resistance to immunotherapy in Multiple Myeloma." Communications Biology 6.1 (2023): 1299. https://doi.org/10.1038/s42003-023-05683-4
The article indicates that although progress has been made in immunotherapy for multiple myeloma, the mechanism of drug resistance remains unclear. This study first proposes that the impairment of the death receptor signaling pathway (especially the FADD/BID axis) is a new mechanism leading to resistance to T-cell immunotherapy, providing a new direction for overcoming resistance.
3. Yang, Liming, et al. "Hypoxia-mediated SUMOylation of FADD exacerbates endothelial cell injury via the RIPK1-RIPK3-MLKL signaling axis." Cell Death & Disease 16.1 (2025): 121. https://doi.org/10.1038/s41419-025-07441-2
This study reveals that hypoxia stabilizes FADD protein through SuMOylation modification and promotes its formation of a complex with RIPK1/RIPK3, thereby inducing necrotic apoptosis in vascular endothelial cells. Inhibiting the SuMOylation of FADD can alleviate this process, providing a new target for the treatment of hypoxic cardiovascular diseases.
4. Zhou, Wenzhao, et al. "The classical apoptotic adaptor FADD regulates glycolytic capacity in acute lymphoblastic leukemia." International Journal of Biological Sciences 18.8 (2022): 3137. https://doi.org/10.7150/ijbs.68016
This study reveals that FADD has a new metabolic regulatory function in T-cell acute lymphoblastic leukemia (T-ALL). The absence of FADD leads to impaired proliferation of Jurkat cells and triggers metabolic reprogramming, weakening their glycolytic capacity and causing them to shift towards mitochondrial respiration. This indicates that FADD is a potential therapeutic target for ALL.
5. Mouasni, Sara, et al. "The classical NLRP3 inflammasome controls FADD unconventional secretion through microvesicle shedding." Cell death & disease 10.3 (2019): 190. https://doi.org/10.1038/s41419-019-1412-9
This study reveals for the first time that in human mononuclear/macrophages, the activation of classical and non-classical NLRP3 inflammasomes can actively induce the secretion of FADD through microbubble shedding, a process independent of pyroptosis. Research has established soluble FADD as a novel biomarker for joint inflammations such as gout and rheumatoid arthritis.
Creative Biolabs: FADD Antibodies for Research
Creative Biolabs specializes in the production of high-quality FADD antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom FADD Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our FADD antibodies, custom preparations, or technical support, contact us at email.
Reference
- Liu, Ying, et al. "FADD as a key molecular player in cancer progression." Molecular Medicine 28.1 (2022): 132. https://doi.org/10.1186/s10020-022-00560-y
Anti-FADD antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ALOX5 Recombinant Antibody (33) (CBMAB-1890CQ)

-
Mouse Anti-DES Monoclonal Antibody (440) (CBMAB-AP1857LY)

-
Rabbit Anti-BRCA2 Recombinant Antibody (D9S6V) (CBMAB-CP0017-LY)

-
Mouse Anti-AKT1 (Phosphorylated S473) Recombinant Antibody (V2-505430) (PTM-CBMAB-0067LY)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

-
Mouse Anti-Acetyl SMC3 (K105/K106) Recombinant Antibody (V2-634053) (CBMAB-AP052LY)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

-
Rat Anti-4-1BB Recombinant Antibody (V2-1558) (CBMAB-0953-LY)

-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

-
Mouse Anti-CFL1 Recombinant Antibody (CBFYC-1771) (CBMAB-C1833-FY)

-
Mouse Anti-ADGRL2 Recombinant Antibody (V2-58519) (CBMAB-L0166-YJ)

-
Mouse Anti-AK4 Recombinant Antibody (V2-180419) (CBMAB-A1891-YC)

-
Mouse Anti-CD24 Recombinant Antibody (SN3) (CBMAB-C1037-CQ)

-
Mouse Anti-CORO1A Recombinant Antibody (4G10) (V2LY-1206-LY806)

-
Mouse Anti-BZLF1 Recombinant Antibody (BZ.1) (CBMAB-AP705LY)

-
Mouse Anti-APOE Recombinant Antibody (A1) (CBMAB-0078CQ)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-CHRNA9 Recombinant Antibody (8E4) (CBMAB-C9161-LY)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Rat Anti-EPO Recombinant Antibody (16) (CBMAB-E1578-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot