FOLR1 Antibodies
Background
The FOLR1 gene encodes a glycoprotein called folate receptor α, which is anchored to the cell membrane by glycosylphosphatidylinositol and is mainly expressed in the central nervous system, kidneys and placenta. It is responsible for binding with high affinity and mediating the uptake of folic acid (vitamin B9) by cells, a process that is crucial for DNA synthesis, methylation reactions and neural development. The dysfunction of this gene is associated with a variety of diseases, including hereditary folic acid absorption disorders and neural tube defects, and has also become a research subject for tumor-targeted therapy. Since its identification in the early 1990s, the crystal structure and folic acid internalization mechanism of FOLR1 have been gradually clarified. Its application research as a biomarker and drug carrier is promoting the development of the field of precision medicine.
Structure of FOLR1
FOLR1 is a glycoprotein with a molecular weight of approximately 28-35 kDa. Its actual measured value varies among species due to different degrees of glycosylation.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 28-35 | 30-32 | 29-31 | 32-34 |
| Primary Structural Differences | High-affinity folic acid binding | High homology with humans | Highly conserved sequence | Glycosylation pattern is slightly different |
This protein is composed of approximately 230 amino acids, and its structure mainly includes a folic acid binding domain. This domain forms a barrel conformation and binds to folic acid molecules with high affinity through multiple key amino acid residues (such as Arg103, Tyr136, Trp140). Its secondary structure is composed of α -helices and β -folds, forming a deep binding pocket that can specifically recognize and capture folic acid molecules. Two conserved disulfide bonds (Cys38-Cys90 and Cys63-Cys192) play a crucial role in maintaining the structural stability of this binding pocket.
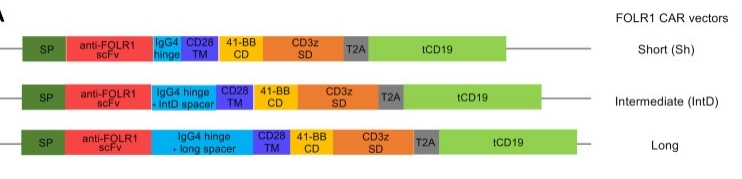 Fig. 1 Engineering a FOLR1-Targeting CAR: Fine-Tuning the IgG4 Spacer Domain.1
Fig. 1 Engineering a FOLR1-Targeting CAR: Fine-Tuning the IgG4 Spacer Domain.1
Key structural properties of FOLR1:
- Barrel shaped tertiary structure constructed by the folate-binding domain
- Deep binding pockets rich in hydrophobic amino acids
- High-affinity folic acid binding sites
Functions of FOLR1
The core function of the FOLR1 protein is to mediate the uptake and transport of folic acid by cells. In addition, it is also involved in a variety of important physiological processes such as cell proliferation regulation, embryonic development, and the maintenance of the homeostasis of the nervous system.
| Function | Description |
| Folic acid intake | Efficiently capture and transport folic acid derivatives such as 5-methyltetrahydrofolate into cells through receptor-mediated endocytosis. |
| Embryonic development | Folic acid is essential for fetal neural tube closure and early organ formation, and its deficiency can lead to neural tube malformations. |
| Nucleotide synthesis | Folate coenzyme, which is necessary for the biosynthesis of purine and thymidylate, maintains normal cell division and DNA replication. |
| Methylation cycle | Participates in one carbon unit metabolism and provides methyl donors for the methylation modification of DNA, proteins and phospholipids. |
| Neuroprotection | Maintaining adequate folic acid levels in the brain supports the synthesis of neurotransmitters and the integrity of myelin sheaths, and affects cognitive function. |
The binding of FOLR1 to folic acid features high affinity and ph-sensitivity. It can effectively release folic acid in slightly acidic environments (such as endosomes), thereby achieving its efficient transmembrane transport cycle. This characteristic is closely related to the targeted delivery design of antibody-drug conjugates (ADCs).
Applications of FOLR1 and FOLR1 Antibody in Literature
1. Zhao, Jiali, et al. "Cancer-associated fibroblasts induce sorafenib resistance of hepatocellular carcinoma cells through CXCL12/FOLR1." BMc cancer 23.1 (2023): 1198. https://doi.org/10.1186/s12885-023-11613-8
The article indicates that in hepatocellular carcinoma, cancer-associated fibroblasts upregulate the expression of FOLR1 in tumor cells by secreting CXCL12, thereby inducing resistance to sorafenib. This study reveals that the CXCL12/FOLR1 pathway is a novel mechanism by which CAFs mediate HCC drug resistance.
2. Matsunaga, Yuki, et al. "Novel anti-FOLR1 antibody–drug conjugate MORAb-202 in breast cancer and non-small cell lung cancer cells." Antibodies 10.1 (2021): 6. https://doi.org/10.3390/antib10010006
This study reveals that the resistance of liver cancer cells to sorafenib is closely related to cancer-associated fibroblasts. This cell mediates the drug resistance process by secreting CXCL12 and upregulating the expression of FOLR1 in liver cancer cells.
3. Huang, Ming-ju, et al. "FOLR1 increases sensitivity to cisplatin treatment in ovarian cancer cells." Journal of Ovarian Research 11.1 (2018): 15. https://doi.org/10.1186/s13048-018-0387-y
This study confirmed that FOLR1 is highly expressed in ovarian cancer, and its expression level is positively correlated with cisplatin sensitivity. Overexpression of FOLR1 can enhance the sensitivity of cancer cells to cisplatin and promote their apoptosis. FOLR1 may serve as a biomarker for improving the efficacy of platinum-based chemotherapy.
4. Wang, Qian, et al. "Case Report: Cerebral folate deficiency caused by FOLR1 variant." Frontiers in Pediatrics 12 (2024): 1434209. https://doi.org/10.3389/fped.2024.1434209
This study reports two cases of brain folic acid deficiency caused by novel FOLR1 gene variations. The child presented with intractable epilepsy, ataxia and developmental regression, and the level of 5-MTHF in cerebrospinal fluid was decreased. The research emphasizes that for such neurological diseases caused by FOLR1 deficiency, early diagnosis is of vital importance.
5. Le, Quy, et al. "CBFA2T3-GLIS2 model of pediatric acute megakaryoblastic leukemia identifies FOLR1 as a CAR T cell target." The Journal of clinical investigation 132.22 (2024). https://doi.org/10.1172/JCI157101
This study developed a CAR-T therapy targeting FOLR1 for acute myeloid leukemia driven by the CBFA2T3-GLIS2 fusion gene. Preclinical experiments have confirmed that this therapy can effectively inhibit C/G leukemia. However, FOLR1 is also expressed in renal and lung tissues, and its potential toxicity is a key issue that needs to be addressed in future clinical applications.
Creative Biolabs: FOLR1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality FOLR1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom FOLR1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our FOLR1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Le, Quy, et al. "CBFA2T3-GLIS2 model of pediatric acute megakaryoblastic leukemia identifies FOLR1 as a CAR T cell target." The Journal of clinical investigation 132.22 (2024). https://doi.org/10.1172/JCI157101
Anti-FOLR1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ACLY Recombinant Antibody (V2-179314) (CBMAB-A0610-YC)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

-
Mouse Anti-ENO2 Recombinant Antibody (H14) (CBMAB-E1341-FY)

-
Mouse Anti-ELAVL4 Recombinant Antibody (6B9) (CBMAB-1132-YC)

-
Mouse Anti-8-oxoguanine Recombinant Antibody (V2-7719) (CBMAB-1898CQ)

-
Mouse Anti-Acetyl SMC3 (K105/K106) Recombinant Antibody (V2-634053) (CBMAB-AP052LY)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

-
Mouse Anti-AK4 Recombinant Antibody (V2-180419) (CBMAB-A1891-YC)

-
Rat Anti-FABP3 Recombinant Antibody (CBXF-2299) (CBMAB-F1612-CQ)

-
Mouse Anti-BCL6 Recombinant Antibody (CBYY-0435) (CBMAB-0437-YY)

-
Mouse Anti-FLI1 Recombinant Antibody (CBXF-0733) (CBMAB-F0435-CQ)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-ESR1 Recombinant Antibody (Y31) (CBMAB-1208-YC)

-
Mouse Anti-ABL2 Recombinant Antibody (V2-179121) (CBMAB-A0364-YC)

-
Rat Anti-CCR2 Recombinant Antibody (475301) (CBMAB-C1338-LY)

-
Mouse Anti-ADGRL2 Recombinant Antibody (V2-58519) (CBMAB-L0166-YJ)

-
Mouse Anti-CD24 Recombinant Antibody (ALB9) (CBMAB-0176CQ)

-
Mouse Anti-CARTPT Recombinant Antibody (113612) (CBMAB-C2450-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








