LCP2 Antibodies
Background
LCP2 is a adaptor protein mainly expressed in immune cells, and its structure consists of the SH2 domain and multiple protein-protein interaction modules. The protein encoded by this gene is involved in the T-cell receptor signal transduction pathway and activates T lymphocytes by regulating the formation of immune synapses and the assembly of signal molecules, which is crucial for adaptive immune responses. After being first identified by a team from Harvard Medical School in 2000, LCP2 has become a research hotspot due to its pivotal role in immune signal transduction, and its mutations are associated with a variety of immune deficiency diseases. As a key signal node protein in the field of immunology, the research on LCP2 has greatly promoted people's understanding of the activation mechanism of immune cells and the signal transduction cascade reaction, and provided a molecular basis for the development of related immunotherapies.
Structure of LCP2
LCP2 is a adaptor protein with a molecular weight of approximately 38-40 kDa. Its precise molecular weight varies slightly among different species, mainly depending on post-translational modifications and splicing variants.
| Species | Human | Mouse | Rat | Rhesus monkey |
| Molecular Weight (kDa) | 38.5 | 39.2 | 38.8 | 38.6 |
| Primary Structural Differences | SH2 domain containing and multiple tyrosine phosphorylation sites | Highly conservative, but the C-end sequence is slightly different | The similarity to human LCP2 is over 90% | Almost homologous to humans |
LCP2 is composed of approximately 350 amino acids, and its structure includes the N-terminal SH2 domain and multiple protein interaction modules at the C-terminal, forming a flexible scaffold structure. The core function of this protein depends on its SH2 domain, which can specifically recognize phosphorylated tyrosine residues, thereby mediating downstream signal transduction of T-cell receptors (TCRS). Its tertiary structure maintains stability through a hydrophobic core, and the phosphorylation of key tyrosine sites (such as Y171 and Y191) can induce conformational changes and promote the binding with signaling molecules such as ZAP-70 and SLP-76. The activity of LCP2 is regulated by dynamic phosphorylation, forming a signal complex at the immune synapse that precisely regulates the T-cell activation threshold.
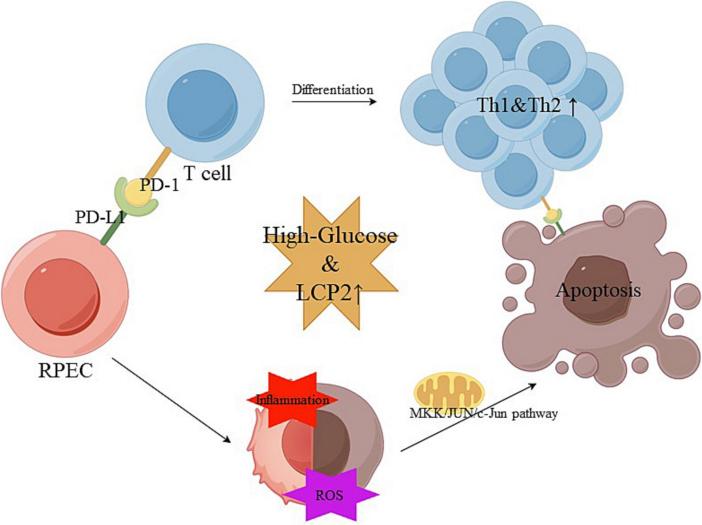 Fig. 1 Diagram illustrating LCP2 mechanisms in diabetic retinopathy.1
Fig. 1 Diagram illustrating LCP2 mechanisms in diabetic retinopathy.1
Key structural properties of LCP2:
- Modular adaptor protein structure
- Phosphotyrosine binding pocket
- Adjustable tyrosine phosphorylation sites
- Flexible connection area
Functions of LCP2
The core function of the LCP2 gene is to serve as a molecular scaffold for the T-cell receptor signaling pathway and simultaneously participate in multiple immune regulatory processes:
| Function | Description |
| Regulation of T cell activation | Signal molecules such as ZAP-70 and SLP-76 were recruited through the SH2 domain to form an immune synaptic signal complex. |
| Signal transduction hub | Integrate the TCR/CD28 co-stimulatory signal to regulate the activation of downstream transcription factors such as NF-κB and NFAT. |
| Maintenance of immune tolerance | Influences thymocyte development by regulating positive/negative selection thresholds. |
| Modulation of inflammatory response | It participates in regulating the production and secretion of cytokines (such as IL-2 and IFN-γ). |
| Prevention and treatment of autoimmunity | The dysfunction and autoimmune diseases such as rheumatoid arthritis, lupus erythematosus (sle). |
The signal transduction of LCP2 exhibits typical "bistable switch" characteristics (compared with the progressive response of linear signaling proteins), and this nonlinear feature enables it to precisely control the T-cell activation threshold, ensuring a rapid immune response while preventing autoimmune damage caused by excessive activation. The modification status of key phosphorylation sites such as Y171/Y191 directly determines the opening/closing of the signaling pathway.
Applications of LCP2 and LCP2 Antibody in Literature
1. Lu, Mingzhi, et al. "Inhibition of LCP2 in T cells alleviated apoptosis and oxidative stress via PD-1/PD-L1 in diabetic retinopathy." International Immunopharmacology 163 (2025): 115240. https://doi.org/10.1016/j.intimp.2025.115240
This study indicates that LCP2, as a platelet-related marker of diabetic retinopathy, promotes Th1 inflammation, oxidative stress and mitochondrial apoptosis by activating the PD-1/PD-L1 signaling pathway, leading to retinal damage. Inhibiting LCP2 or PD-1/PD-L1 can significantly improve the lesion, and combined treatment enhances the efficacy. Targeting this axis may become a new strategy for DR Treatment.
2. Yu, Ya-Li, et al. "STAT1 epigenetically regulates LCP2 and TNFAIP2 by recruiting EP300 to contribute to the pathogenesis of inflammatory bowel disease." Clinical epigenetics 13.1 (2021): 127. https://doi.org/10.1186/s13148-021-01101-w
Research has found that STAT1 activates the expression of LCP2 and TNFAIP2 through EP300-mediated H3K27ac modification, promoting the development of inflammatory bowel disease (IBD). Inhibition of EP300 can alleviate colitis, suggesting that the STAT1-EP300-H3K27ac-LCP2 axis may be a new target for the treatment of IBD.
3. Wang, Zijun, and Mou Peng. "A novel prognostic biomarker LCP2 correlates with metastatic melanoma-infiltrating CD8+ T cells." Scientific reports 11.1 (2021): 9164. https://doi.org/10.1038/s41598-021-88676-9
Research has found that LCP2 is highly expressed in metastatic melanoma, which is associated with a favorable prognosis and increased infiltration of CD8+ T cells. It participates in the regulation of TCR signaling and immune checkpoints, and may serve as a predictive marker for anti-PD1 therapy and a new target for immunotherapy.
4. Hu, Qin, et al. "Value of altered methylation patterns of genes RANBP3, LCP2 and GRAP2 in cfDNA in breast cancer diagnosis." Journal of Medical Biochemistry 43.3 (2024): 387. https://doi.org/10.5937/jomb0-47507
Research has found that the methylation level of the LCP2 gene is significantly altered in breast cancer tissues and is associated with the malignancy of the tumor. The diagnostic efficacy of LCP2 methylation detection in plasma cfDNA reached 83.9%, suggesting that it can be used as a potential liquid biopsy marker for the auxiliary diagnosis of breast cancer.
5. Shen, nyang, et al. "Using weighted gene co-expression network analysis to identify key genes related to preeclampsia." Frontiers in Immunology 16 (2025): 1569591. https://doi.org/10.3389/fimmu.2025.1569591
Research has found that LCP2 is associated with the pathogenesis of preeclampsia, and its protein expression is significantly down-regulated in the placenta of patients. As a core gene of immune-related pathways, LCP2 may become a potential diagnostic marker for preeclampsia.
Creative Biolabs: LCP2 Antibodies for Research
Creative Biolabs specializes in the production of high-quality LCP2 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom LCP2 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our LCP2 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Lu, Mingzhi, et al. "Inhibition of LCP2 in T cells alleviated apoptosis and oxidative stress via PD-1/PD-L1 in diabetic retinopathy." International Immunopharmacology 163 (2025): 115240. https://doi.org/10.1016/j.intimp.2025.115240
Anti-LCP2 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-BRD3 Recombinant Antibody (CBYY-0801) (CBMAB-0804-YY)

-
Mouse Anti-AZGP1 Recombinant Antibody (CBWJZ-007) (CBMAB-Z0012-WJ)

-
Rabbit Anti-CBL Recombinant Antibody (D4E10) (CBMAB-CP0149-LY)

-
Mouse Anti-C5b-9 Recombinant Antibody (aE11) (CBMAB-AO138LY)

-
Mouse Anti-ADRB2 Recombinant Antibody (V2-180026) (CBMAB-A1420-YC)

-
Mouse Anti-2C TCR Recombinant Antibody (V2-1556) (CBMAB-0951-LY)

-
Mouse Anti-ARSA Recombinant Antibody (CBYC-A799) (CBMAB-A3679-YC)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-AQP2 Recombinant Antibody (G-3) (CBMAB-A3359-YC)

-
Mouse Anti-ARHGDIA Recombinant Antibody (CBCNA-009) (CBMAB-R0415-CN)

-
Mouse Anti-BACE1 Recombinant Antibody (61-3E7) (CBMAB-1183-CN)

-
Mouse Anti-CGAS Recombinant Antibody (CBFYM-0995) (CBMAB-M1146-FY)

-
Mouse Anti-ACKR3 Recombinant Antibody (V2-261265) (CBMAB-C1023-LY)

-
Rabbit Anti-ALK (Phosphorylated Y1278) Recombinant Antibody (D59G10) (PTM-CBMAB-0035YC)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Mouse Anti-CARD11 Recombinant Antibody (CBFYC-0811) (CBMAB-C0866-FY)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






