LDHA Antibodies
Background
LDHA is a key metabolic enzyme protein, mainly found in the skeletal muscles and other high-energy-consuming tissues of vertebrates. This enzyme catalyzes the reverse reaction of pyruvate converting to lactic acid, maintaining cellular energy supply and REDOX balance during anaerobic glycolysis. During intense exercise, muscle tissue rapidly regenerates NAD+ through LDHA to ensure continuous glycolysis function. In the middle of the 20th century, scientists systematically analyzed its tetramer structure and found that its subunit composition was tissue-specific. This enzyme is not only the core molecule of tumor metabolic reprogramming (Warburg effect), but its isoenzyme conversion mechanism has also deepened people's understanding of metabolic adaptation, disease occurrence and evolutionary regulation, providing an important theoretical basis for targeted therapy research.
Structure of LDHA
LDHA is a glycolytic enzyme of approximately 36 kDa, and its molecular weight varies slightly among different species:
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 36.4 | 36.2 | 36.3 | 36.5 |
| Primary Structural Differences | 332 amino acids | 331 amino acids | 332 amino acids | 333 amino acids |
The LDHA protein is composed of four subunits, each of which contains the classic Rossmann folding domain. The key residues in the active center include arginine 109, histidine 193 and aspartic acid 168, which together form the NADH binding site. The catalytic mechanism of this enzyme relies on the synergistic effect of these conserved amino acids, enabling it to reversibly catalyze the conversion between pyruvate and lactic acid. The stability of the quaternary structure of LDHA is closely related to its biological functions, especially playing a key role in maintaining the REDOX balance of cells.
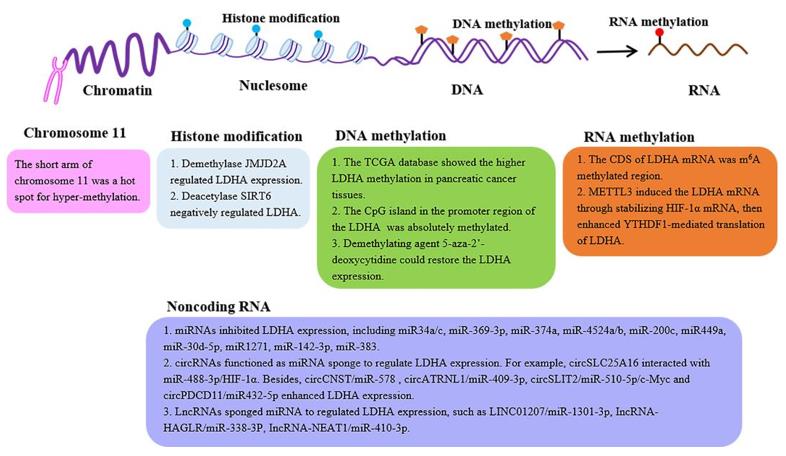 Fig. 1 The epigenetic regulation of the LDHA expression.1
Fig. 1 The epigenetic regulation of the LDHA expression.1
Key structural properties of LDHA:
- Classic Rossmann folding structure responsible for NAD +
- The highly conserved active catalytic pocket is located at the junction of α-helix and β-fold
- Four dimer interface by hydrophobic interactions and hydrogen bonding network stability
Functions of LDHA
The core function of LDHA is to catalyze the final steps of glycolysis and simultaneously participate in multiple cellular metabolic regulation processes:
| Function | Description |
| Anaerobic glycolysis | Under hypoxic conditions, pyruvate is reduced to lactic acid, while NAD+ is regenerated to maintain glycolytic flux. |
| Cellular pH regulation | Through the production of lactic acid, it participates in the maintenance of intracellular acid-base balance and affects the metabolic microenvironment. |
| Warburg effect | High expression in tumor cells, and promote the characteristic aerobic glycolysis metabolic reprogramming. |
| REDOX steady state | Regulate the cytoplasmic NAD+/NADH ratio and affect multiple REDOX sensitive signaling pathways. |
| Metabolite signal transduction | Lactic acid, as a signaling molecule, regulates epigenetic modifications such as histone lactation. |
The equilibrium constant of the reaction catalyzed by LDHA is far biased towards lactic acid production (Keq≈2.5×10^4). This thermodynamic property enables it to rapidly drive metabolism towards lactic acid accumulation under hypoxia, forming a typical metabolic branch regulation with the aerobic metabolic pathway of pyruvate dehydrogenase.
Applications of LDHA and LDHA Antibody in Literature
1. Tang, Yu, et al. "LDHA: The Obstacle to T cell responses against tumor." Frontiers in Oncology 12 (2022): 1036477. https://doi.org/10.3389/fonc.2022.1036477
This study reveals that lactate dehydrogenase A (LDHA), as a key enzyme in glycolysis, regulates its expression through epigenetic mechanisms, influencing the metabolic reprogramming of tumor cells and T cells, thereby limiting the efficacy of immunotherapy. This article reviews the current status of LDHA inhibitors and their combined strategies with immunotherapy, providing new ideas for future treatment plans.
2. Zhang, Kun, et al. "N6-methyladenosine-mediated LDHA induction potentiates chemoresistance of colorectal cancer cells through metabolic reprogramming." Theranostics 12.10 (2022): 4802. https://doi.org/10.7150/thno.73746
This study reveals that LDHA is a key enzyme regulating tumor and T-cell metabolism, and its epigenetic modifications can promote immune escape and affect the efficacy of immunotherapy. Inhibiting LDHA or combining it with immunotherapy is a promising anti-cancer strategy for the future.
3. Yang, Hao, et al. "Glucose transporter 3 (GLUT3) promotes lactylation modifications by regulating lactate dehydrogenase A (LDHA) in gastric cancer." Cancer Cell International 23.1 (2023): 303. https://doi.org/10.1186/s12935-023-03162-8
This study reveals that GLUT3 affects histone lactation modification by regulating LDHA expression, thereby driving the invasion and metastasis of gastric cancer. Targeting the GLUT3/LDHA/ lactation axis can inhibit the progression of gastric cancer, providing a new target for the diagnosis and treatment of this disease.
4. Yu, Yuanhang, et al. "LRPPRC promotes glycolysis by stabilising LDHA mRNA and its knockdown plus glutamine inhibitor induces synthetic lethality via m6A modification in triple‐negative breast cancer." Clinical and translational medicine 14.2 (2024): e1583. https://doi.org/10.1002/ctm2.1583
This study found that in triple-negative breast cancer, the m6A reader LRPPRC promotes glycolysis and tumor progression by recognizing and enhancing the stability of LDHA mRNA. Inhibiting LRPPRC can trigger metabolic reprogramming. At this point, combined blocking of glutaminase can achieve the therapeutic effect of "synthetic lethargy".
5. Juanjuan, M. E. N. G., Y. A. N. Caiwen, and L. I. U. Jinchun. "LDHA-Mediated Histone Lactylation Promotes the Nonalcoholic Fatty Liver Disease Progression Through Targeting The METTL3/YTHDF1/SCD1 m6A Axis." Physiological Research 73.6 (2024): 985. https://doi.org/10.33549/physiolres.935289
This study reveals that in non-alcoholic fatty liver disease, histone lactation modification (H3K18lac) catalyzed by LDHA can up-regulate the expression of METTL3. METTL3 then stabilizes SCD1 mRNA through the m6A-YTHDF1 mechanism, ultimately driving liver lipid deposition and disease progression.
Creative Biolabs: LDHA Antibodies for Research
Creative Biolabs specializes in the production of high-quality LDHA antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom LDHA Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our LDHA antibodies, custom preparations, or technical support, contact us at email.
Reference
- Tang, Yu, et al. "LDHA: The Obstacle to T cell responses against tumor." Frontiers in Oncology 12 (2022): 1036477. https://doi.org/10.3389/fonc.2022.1036477
Anti-LDHA antibodies
 Loading...
Loading...
Hot products 
-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-ARSA Recombinant Antibody (CBYC-A799) (CBMAB-A3679-YC)

-
Mouse Anti-CARTPT Recombinant Antibody (113612) (CBMAB-C2450-LY)

-
Mouse Anti-BSN Recombinant Antibody (219E1) (CBMAB-1228-CN)

-
Mouse Anti-CD83 Recombinant Antibody (HB15) (CBMAB-C1765-CQ)

-
Mouse Anti-ATM Recombinant Antibody (2C1) (CBMAB-A3970-YC)

-
Mouse Anti-BLNK Recombinant Antibody (CBYY-0623) (CBMAB-0626-YY)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Mouse Anti-CFL1 Recombinant Antibody (CBFYC-1771) (CBMAB-C1833-FY)

-
Mouse Anti-FLI1 Recombinant Antibody (CBXF-0733) (CBMAB-F0435-CQ)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-EPO Recombinant Antibody (CBFYR0196) (CBMAB-R0196-FY)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Mouse Anti-ALX1 Recombinant Antibody (96k) (CBMAB-C0616-FY)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

-
Mouse Anti-ALPL Antibody (B4-78) (CBMAB-1009CQ)

-
Mouse Anti-APCS Recombinant Antibody (CBYC-A663) (CBMAB-A3054-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








