LTBR Antibodies
Background
LTBR gene encoding lymphotoxin beta receptors, this is a kind of on the surface of the cell tumor necrosis factor receptor superfamily member, mainly expressed in the middle part of the immune cells and stromal cells. This receptor plays a core role in the development of secondary lymphoid organs, the recruitment of immune cells and the regulation of inflammatory responses by binding to the lymphotoxin α1β2 heterotrimer and activating the NF-κB and MAPK signaling pathways. First identified by Browning's team in 1991, the discovery of LTBR revealed the unique mechanism of the tumor necrosis factor family in adaptive immunity, and its three-dimensional structure analysis provided an important target for the treatment of autoimmune diseases and cancer. The functional research of this gene has greatly advanced our understanding of the regulation of the immune microenvironment, the mechanism of chronic inflammation, and the maintenance of lymphoid tissue homeostasis. Currently, targeted drugs for LTBR have entered the clinical trial stage for multiple autoimmune diseases.
Structure of LTBR
LTBR is a transmembrane protein with a molecular weight of approximately 25-30 kDa, and its precise molecular weight varies slightly depending on the degree of glycosylation modification.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | 28-30 | 25-27 | 26-28 |
| Primary Structural Differences | Containing TNF receptor characteristic domain (CRD1-4) | The intracellular segment signal modons are slightly different | With the human LTBR 80% homology |
LTBR is composed of 435 amino acids, and its extracellular region contains four cysteine-rich domains (CRDS), which are responsible for binding to the ligand lymphotoxin α1β2. Maintain receptor anchoring in the transmembrane region; The intracellular segment recruits and activates downstream signaling pathways (such as NF-κB) through TRAF proteins. The tertiary structure of this protein presents a typical folding pattern of the TNF receptor superfamily, in which the key glutamate residue (Glu142) in the CRD3 domain is directly involved in ligand recognition. Post-translational modifications (such as N-glycosylation) can affect its membrane localization and signal transduction efficiency, while disulfide bond networks (such as Cys108-Cys119) stabilize its extracellular conformation.
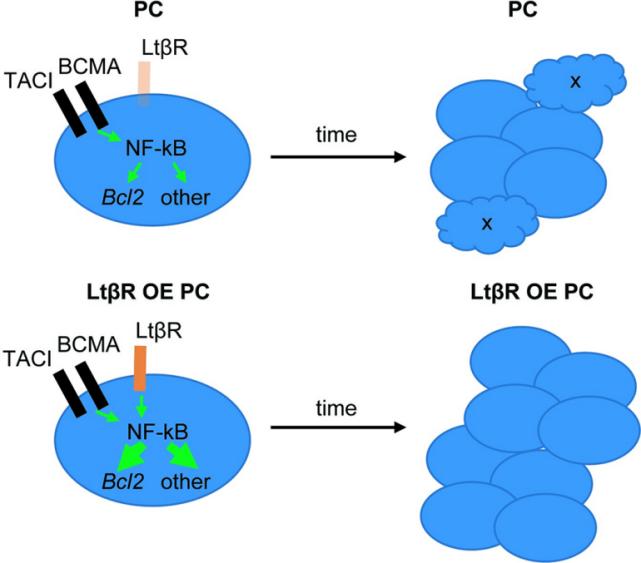 Fig. 1 Model for LTβR over-expression (OE) effect on PC accumulation.1
Fig. 1 Model for LTβR over-expression (OE) effect on PC accumulation.1
Key structural properties of LTBR:
- TNF receptor feature folding
- Ligand-binding pocket
- Transmembrane signal transduction module
- Intracellular signal transduction motifs
- Disulfide bond network
Functions of LTBR
The core function of LTBR is to regulate the development of the immune system and inflammatory responses, and it also plays a key role in various pathological processes.
| Function | Description |
| Development of secondary lymphoid organs | By activating the NF-κB signaling pathway, it promotes the formation and structural maintenance of lymphoid tissues such as lymph nodes and spleen. LTBR deficiency can lead to lymphoid organ hypoplasia (such as the absence of lymph nodes in LTα/β knockout mice). |
| Recruitment and localization of immune cells | Regulate the expression of chemokines (such as CXCL13 and CCL21), and guide the directional migration and aggregation of B cells, T cells and dendritic cells in lymphoid tissue. |
| Regulation of inflammatory response | In chronic inflammation (such as rheumatoid arthritis), excessive activation of LTBR signaling can lead to abnormal secretion of pro-inflammatory cytokines (TNF-α, IL-6). |
| Anti-tumor immune response | T cell infiltration and activity in the tumor microenvironment can be enhanced by maintaining lymphoid structures (such as tertiary lymphoid structures, TLS), but their overexpression may also promote the progression of lymphoma. |
| Regulation of apoptosis | Under certain conditions (such as the TRAF2-dependent pathway), LTBR can trigger pro-apoptotic signals and eliminate abnormal immune cells. |
Signaling pathway characteristics: After binding to the ligand lymphotoxin α1β2, LTBR preferentially activates the classical NF-κB pathway (via TRAF2/5), while its association with non-classical NF-κB pathways (via NIK/IKKα) is relatively weak. This selective signal transduction endows it with precise regulatory capabilities in immune homeostasis.
Applications of LTBR and LTBR Antibody in Literature
1. Hu, Zhengyun, and Guo Zhou. "CREB1 Transcriptionally Activates LTBR to Promote the NF-κB Pathway and Apoptosis in Lung Epithelial Cells." Computational and Mathematical Methods in Medicine 2022.1 (2022): 9588740. https://doi.org/10.1155/2022/9588740
Research has found that LTBR activates and regulates the NF-κB pathway through CREB1 transcription in bronchopulmonary dysplasia (BPD), promoting apoptosis of lung epithelial cells. Silencing LTBR can enhance cell survival and provide a new target for the treatment of BPD.
2. Wang, Liang, et al. "LTBR acts as a novel immune checkpoint of tumor-associated macrophages for cancer immunotherapy." Imeta 3.5 (2024): e233. https://doi.org/10.1002/imt2.233
Research has found that lymphotoxin beta receptor (LTBR) is a novel immune checkpoint for tumor-associated macrophages (Tams) in lung adenocarcinoma, and its high expression is associated with a poor prognosis. LTBR maintains the immunosuppressive function of Tams through the NF-κB and Wnt/β-catenin pathways. Targeted silencing of LTBR can enhance the effect of immunotherapy.
3. Wu, Yinteng, et al. "Systematic analysis of the prognostic value and immunological function of LTBR in human cancer." Aging (Albany NY) 16.1 (2024): 129. https://doi.org/10.18632/aging.205356
Research has found that LTBR is highly expressed in various tumors and predicts a poor prognosis. It is closely related to immune cell infiltration, tumor stem cell characteristics, and immunomodulatory genes, and can serve as a potential therapeutic target and biomarker for immunotherapy.
4. Bista, Pradeep, et al. "TRAF3 controls activation of the canonical and alternative NFκB by the lymphotoxin beta receptor." Journal of Biological Chemistry 285.17 (2010): 12971-12978. https://doi.org/10.1074/jbc.m109.076091
Research has found that TRAF3 negatively regulates LTBR signaling through a dual mechanism: inhibiting the activation of the classical NF-κB pathway (reducing TRAF2/IKK1 recruitment), and simultaneously suppressing the spontaneous activation of non-classical NF-κB pathways (maintaining p100/RelB/NIK homeostasis). Regulating the TRAF3 level can balance the dual-path signal transduction of LTBR.
5. Wu, Guolin, et al. "Silencing of TRAF5 enhances necroptosis in hepatocellular carcinoma by inhibiting LTBR-mediated NF-κB signaling." PeerJ 11 (2023): e15551. https://doi.org/10.7717/peerj.15551
Research has found that TRAF5 promotes the proliferation and metastasis of liver cancer cells and inhibits necrotic apoptosis by activating the NF-κB signaling pathway through LTBR. Targeting the TRAF5/LTBR/NF-κB axis can inhibit tumor growth and induce cell death, providing a new strategy for the treatment of liver cancer.
Creative Biolabs: LTBR Antibodies for Research
Creative Biolabs specializes in the production of high-quality LTBR antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom LTBR Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our LTBR antibodies, custom preparations, or technical support, contact us at email.
Reference
- Kotov, Jessica A., et al. "LTβR overexpression promotes plasma cell accumulation." Plos one 17.8 (2022): e0270907. https://doi.org/10.1371/journal.pone.0270907
Anti-LTBR antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CARTPT Recombinant Antibody (113612) (CBMAB-C2450-LY)

-
Mouse Anti-BIRC3 Recombinant Antibody (16E63) (CBMAB-C3367-LY)

-
Mouse Anti-BLNK Recombinant Antibody (CBYY-0623) (CBMAB-0626-YY)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-AZGP1 Recombinant Antibody (CBWJZ-007) (CBMAB-Z0012-WJ)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Mouse Anti-ALOX5 Recombinant Antibody (33) (CBMAB-1890CQ)

-
Mouse Anti-CD24 Recombinant Antibody (ALB9) (CBMAB-0176CQ)

-
Mouse Anti-CECR2 Recombinant Antibody (CBWJC-2465) (CBMAB-C3533WJ)

-
Mouse Anti-B2M Recombinant Antibody (CBYY-0050) (CBMAB-0050-YY)

-
Rat Anti-ABCC11 Recombinant Antibody (V2-179001) (CBMAB-A0236-YC)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Mouse Anti-DDC Recombinant Antibody (8E8) (CBMAB-0992-YC)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-AOC3 Recombinant Antibody (CBYY-0014) (CBMAB-0014-YY)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503416) (CBMAB-V208-1402-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








