MANF Antibodies
Background
MANF is a secretory endoplasmic reticulum stress response protein, mainly distributed in the central nervous system and endocrine tissues. This protein exerts neuroprotective effects by regulating the unfolded protein response (UPR) pathway, can inhibit endoplasmic reticulum stress-induced apoptosis, and has shown therapeutic potential in disease models such as Parkinson's disease and diabetes. MANF was first identified by the Finnish scientist Lindholm's team in 2003 while studying midbrain dopaminergic neurons. It is the first neuroprotective factor discovered to have a unique structure (containing SAP-like domains rather than typical neurotrophic factor structures). Its unique mechanism of action breaks through the cognitive framework of the traditional neurotrophic factor family, providing new molecular targets for the treatment strategies of neurodegenerative diseases and metabolic diseases.
Structure of MANF
The main function of MANF is to regulate cellular stress responses and neuroprotection, while also participating in various pathophysiological processes.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | ~18-20 | ~18-19 | ~19-20 |
| Primary Structural Differences | Contains SAP-like domain and conserved C terminus | Highly homologous and functionally similar | Highly homologous to rat CDNF |
MANF is composed of 182 amino acids, and its three-dimensional structure contains a unique SAP-like domain (mediating lipid binding) and a conserved C-terminal domain (responsible for endoplasmic reticulum stress response). This protein does not contain typical neurotrophic factor structures but exerts neuroprotective effects by regulating the unfolded protein response (UPR). Its activity depends on the disulfide bond formed by two key cysteine residues (Cys157 and Cys68), which is crucial for maintaining protein stability. The secondary structure of MANF is mainly composed of α -helices and β -folds, forming a hydrophobic core to bind stress-related signaling molecules.
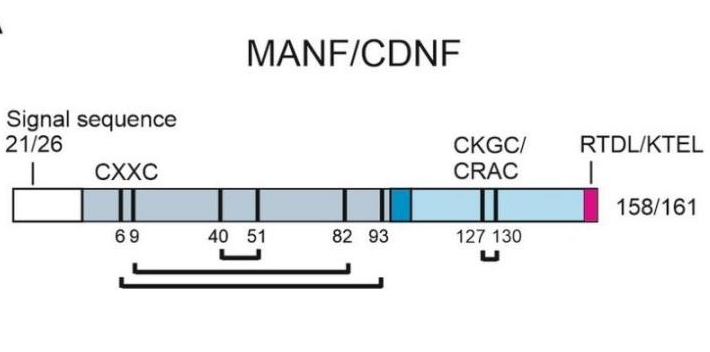 Fig. 1 Structure of human MANF and CDNF proteins.1
Fig. 1 Structure of human MANF and CDNF proteins.1
Key structural properties of MANF:
- Unique SAP-like domain
- Conservative C-end domain structure
- Disulfide bond-dependent stability
- Without the typical structure of neurotrophic factors
- Acid-base sensitive structure
Functions of MANF
The main function of MANF is to regulate cellular stress responses and neuroprotection, while also participating in various pathophysiological processes.
| Function | Description |
| Neuroprotection | Protect dopaminergic neurons from degenerative damage by inhibiting the apoptotic pathway induced by endoplasmic reticulum stress. |
| Endoplasmic reticulum stress regulation | Regulating unfolded protein response (UPR), maintaining endoplasmic reticulum homeostasis, and playing a protective role in metabolic diseases such as diabetes. |
| Inflammatory regulation | Inhibit pro-inflammatory signaling pathways such as NF-κB to alleviate neuroinflammation and ischemia-reperfusion injury. |
| Vascular repair | Promote the survival of vascular endothelial cells and angiogenesis, and improve tissue repair in ischemic diseases. |
| Metabolic regulation | By regulating the function of islet β cells, it affects insulin secretion and participates in the treatment of diabetes. |
The mechanism of action of MANF does not rely on traditional neurotrophic factor receptors (such as RET), but exerts its function by directly binding to endoplasmic reticulum stress sensors (such as IRE1α) or cell surface receptors (such as SORT1). Its function has significant cell type specificity, and there are differences in the regulatory mechanisms in the central nervous system and the pancreas.
Applications of MANF and MANF Antibody in Literature
1. Kim, Yeawon, et al. "MANF stimulates autophagy and restores mitochondrial homeostasis to treat autosomal dominant tubulointerstitial kidney disease in mice." Nature communications 14.1 (2023): 6493. https://doi.org/10.1038/s41467-023-42154-0
Studies have found that secretory endoplasmic reticulum protein MANF can improve renal tubular injury and fibrosis in hereditary kidney disease ADTKD-UMOD by activating p-AMPK to enhance autophagy/mitochondrial autophagy, eliminate mutant UMOD protein and promote mitochondrial biosynthesis. MANF is expected to become a novel biological therapy for treating protein misfolding diseases.
2. Lõhelaid, Helike, et al. "UPR responsive genes Manf and Xbp1 in stroke." Frontiers in Cellular Neuroscience 16 (2022): 900725. https://doi.org/10.3389/fncel.2022.900725
Research has found that the endoplasmic reticulum stress response factor MANF exerts a neuroprotective effect after stroke, promoting cell survival by regulating neurogenesis and inflammatory responses. Compared with the UPR-related gene XBP1, MANF has unique secretion characteristics and pro-survival mechanisms. Exogenous administration of MANF protein may become a new strategy to improve the prognosis of stroke.
3. Zhang, Caixia, et al. "Navigating the landscape of MANF research: a scientometric journey with citespace analysis." Cellular and Molecular Neurobiology 43.8 (2023): 3897-3913. https://doi.org/10.1007/s10571-023-01412-x
Research findings indicate that bibliometric analysis based on CiteSpace shows a rapid growth trend in MANF research over the past 25 years, with research hotspots expanding from neuroprotective effects to disease therapeutic potential. China and the United States take the lead in this field, promoting the transformation of MANF from basic mechanisms to clinical applications.
4. Kovaleva, Vera, et al. "MANF regulates neuronal survival and UPR through its ER-located receptor IRE1α." Cell reports 42.2 (2023). https://doi.org/10.1016/j.celrep.2023.112066
Research has found that MANF regulates the unfolded protein response (UPR) signaling pathway by directly binding to IRE1α and competitively replacing BiP, thereby inhibiting the oligomerization and phosphorylation of IRE1α. This interaction is crucial for the neuronal protective effect of MANF in Parkinson's disease models, revealing a new mechanism by which it exerts cellular protective functions through IRE1α.
5. Pakarinen, Emmi, and Päivi Lindholm. "CDNF and MANF in the brain dopamine system and their potential as treatment for Parkinson's disease." Frontiers in psychiatry 14 (2023): 1188697. https://doi.org/10.3389/fpsyt.2023.1188697
Research has found that endogenous MANF exerts neuroprotective and restorative effects in Parkinson's disease models by regulating endoplasmic reticulum stress response (UPR) and inflammatory pathways. MANF not only promotes the survival of dopamine neurons but also participates in maintaining protein homeostasis. Its dual mechanism of action provides a new target for the treatment of PD.
Creative Biolabs: MANF Antibodies for Research
Creative Biolabs specializes in the production of high-quality MANF antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MANF Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MANF antibodies, custom preparations, or technical support, contact us at email.
Reference
- Pakarinen, Emmi, and Päivi Lindholm. "CDNF and MANF in the brain dopamine system and their potential as treatment for Parkinson's disease." Frontiers in psychiatry 14 (2023): 1188697. https://doi.org/10.3389/fpsyt.2023.1188697
Anti-MANF antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-APCS Recombinant Antibody (CBYC-A663) (CBMAB-A3054-YC)

-
Rat Anti-4-1BB Recombinant Antibody (V2-1558) (CBMAB-0953-LY)

-
Mouse Anti-ADIPOR1 Recombinant Antibody (V2-179982) (CBMAB-A1368-YC)

-
Mouse Anti-CD24 Recombinant Antibody (2Q1282) (CBMAB-C1624-CN)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Mouse Anti-AMACR Recombinant Antibody (CB34A) (CBMAB-CA034LY)

-
Mouse Anti-BAX Recombinant Antibody (CBYY-0216) (CBMAB-0217-YY)

-
Mouse Anti-AZGP1 Recombinant Antibody (CBWJZ-007) (CBMAB-Z0012-WJ)

-
Mouse Anti-ALB Recombinant Antibody (V2-180650) (CBMAB-A2186-YC)

-
Mouse Anti-CA9 Recombinant Antibody (CBXC-2079) (CBMAB-C0131-CQ)

-
Mouse Anti-ELAVL4 Recombinant Antibody (6B9) (CBMAB-1132-YC)

-
Mouse Anti-CD164 Recombinant Antibody (CBFYC-0077) (CBMAB-C0086-FY)

-
Mouse Anti-APOE Recombinant Antibody (A1) (CBMAB-0078CQ)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-CCND2 Recombinant Antibody (DCS-3) (CBMAB-G1318-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






