MMP2 Antibodies
Background
MMP2 (Matrix metalloproteinase 2) belongs to the zinc-dependent endopeptidase family and is mainly distributed in connective tissues, immune cells and vascular interfaces. The protease encoded by this gene can specifically degrade extracellular matrix components such as type IV collagen, thereby regulating physiological processes such as tissue remodeling, wound repair and angiogenesis. In the tumor microenvironment, MMP2 drives cancer progression by activating growth factors and promoting metastasis pathways. Its three-dimensional structure was analyzed by X-ray crystallography in 1994, revealing a characteristic module architecture composed of a propeptide domain, a catalytic domain and a hemoglobin-like domain. The continuous research on MMP2 not only deepens our understanding of the dynamic balance of extracellular matrix, but also provides a key molecular basis for the development of targeted anti-cancer drugs.
Structure of MMP2
Myoglobin is a relatively small protein with a molecular weight of approximately 16.7 kDa. This weight may slightly vary between species due to minor differences in amino acid sequence.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 72 | 74 | 73 | 72 |
| Primary Structural Differences | Signal peptide, peptide, catalytic domain and hemoglobin before combining domain | Highly conserved catalytic domain | The propeptide region has specific enzyme cleavage sites | Domain structure of highly homologous with humans |
MMP2 is composed of 574 amino acids, and its primary structure folds to form three characteristic domains: the propeptide region, the catalytic domain, and the heme binding domain. The catalytic center contains a zinc ion active site maintained by three histidine residue coordination bonds, which is the core of its proteolytic function. The light-colored appearance of this enzyme stems from its protein nature that does not contain chromophores. Its secondary structure is composed of a mixture of β -lamellar and α -helical layers, and the catalytic domain forms an ellipsoidal structure, providing binding pockets for the substrate. Zinc ions located in the active center directly participate in the hydrolysis of peptide bonds, while structural calcium ions play a role in stabilizing the three-dimensional conformation of proteases.
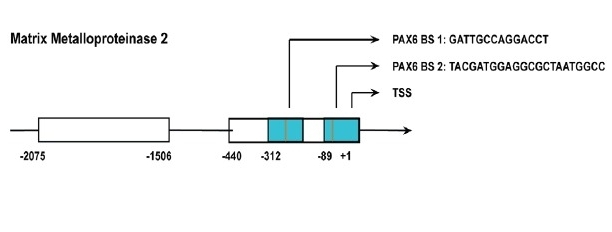 Fig. 1 Structure of the MMP2 gene.1
Fig. 1 Structure of the MMP2 gene.1
Key structural properties of MMP2:
- Multi-domain protease configuration
- The catalytic center contains zinc ion active site
- Calcium ion binding sites maintain structural stability
Functions of MMP2
The core function of the protein encoded by the MMP2 gene is to degrade the extracellular matrix, and it also participates in the regulation of various physiological and pathological processes.
| Function | Description |
| Matrix degradation | Specifically hydrolyze the main components of the basement membrane such as type IV collagen and gelatin to create space for cell migration. |
| Organizational Reshaping | Regulate the reconstruction and renewal of connective tissue during wound healing, embryonic development and bone growth. |
| Angiogenesis | By cutting the matrix to release the encapsulated angiogenic factors (such as VEGF), it promotes the formation of new blood vessels. |
| Immune regulation | By processing cytokines and chemokine precursors, inflammatory responses and immune cell infiltration are regulated. |
| Disease progression | In pathological processes such as tumor invasion, metastasis and arthritis, overactivated MMP2 can disrupt tissue homeostasis. |
The enzymatic activity mechanics of MMP2 follows the typical Michaelis-Menten model, and its catalytic efficiency is strictly regulated by the tissue inhibitor TIMP. This fine balance ensures its dual role in physiological repair and pathological destruction.
Applications of MMP2 and MMP2 Antibody in Literature
1. Muniz-Bongers, Luciana R., et al. "MMP2 and TLRs modulate immune responses in the tumor microenvironment." JCI insight 6.12 (2021): e144913. https://doi.org/10.1172/jci.insight.144913
The article indicates that in melanoma, MMP2 promotes the formation of an immunosuppressive microenvironment through the TLR2/4 signaling pathway, thereby accelerating tumor growth. Its overexpression leads to a reduction in cytotoxic cells and an increase in M2 macrophages, while knockdown of MMP2 can enhance the anti-tumor immunity of T cells and NK cells. This effect depends on the interaction between Batf3 and RAG2-mediated DC-T cells.
2. Han, Liping, et al. "Correlation between MMP2 expression in lung cancer tissues and clinical parameters: a retrospective clinical analysis." BMC pulmonary medicine 20.1 (2020): 283. https://doi.org/10.1186/s12890-020-01317-1
This study shows that the expression of MMP2 in lung cancer tissues is significantly elevated and is closely related to poor tumor differentiation, lymph node metastasis, advanced stage and poor prognosis. MMP2 is an independent prognostic factor for lung cancer and can serve as a potential diagnostic and prognostic biomarker.
3. Ruiz-Gómez, Gloria, et al. "Glycosaminoglycans influence enzyme activity of MMP2 and MMP2/TIMP3 complex formation-insights at cellular and molecular level." Scientific Reports 9.1 (2019): 4905. https://doi.org/10.1038/s41598-019-41355-2
This study reveals that glycosaminoglycans (GAGs) such as sulfated hyaluronic acid can regulate the activity of MMP2 and the formation of its complex with the inhibitor TIMP3. Molecular simulations have shown that GAG influences MMP2 function by stabilizing protein-protein interactions, providing a new perspective for precise intervention in extracellular matrix remodeling.
4. Bornschein, Jan, et al. "MMP2 and MMP7 at the invasive front of gastric cancer are not associated with mTOR expression." Diagnostic Pathology 10.1 (2015): 212. https://doi.org/10.1186/s13000-015-0449-z
This study shows that MMP2 is more expressed at the tumor invasion frontier in gastric cancer, but its expression has no significant association with the mTOR signaling pathway. Rapamycin's inhibition of mTOR phosphorylation did not affect MMP expression, indicating that MMP2 may be regulated by other mechanisms in gastric cancer.
5. Asghar, Muhammad Yasir, et al. "Sphingosine 1-phosphate attenuates MMP2 and MMP9 in human anaplastic thyroid cancer C643 cells: Importance of S1P2." PLoS One 13.5 (2018): e0196992. https://doi.org/10.1371/journal.pone.0196992
This study shows that in thyroid cancer C643 cells, S1P inhibits calproteinase activity by activating the S1P2 receptor and the Rho-ROCK pathway, thereby significantly reducing the expression, secretion and activity of MMP2, and ultimately weakening the invasion ability of tumor cells.
Creative Biolabs: MMP2 Antibodies for Research
Creative Biolabs specializes in the production of high-quality MMP2 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MMP2 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MMP2 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Urrutia, Guillermo, et al. "PAX6 promoter methylation correlates with MDA-MB-231 cell migration, and expression of MMP2 and MMP9." Asian Pacific Journal of Cancer Prevention: APJCP 19.10 (2018): 2859. https://doi.org/10.22034/APJCP.2018.19.10.2859
Anti-MMP2 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-Acetyl-α-Tubulin (Lys40) Recombinant Antibody (V2-623485) (CBMAB-CP2897-LY)

-
Mouse Anti-ARHGAP5 Recombinant Antibody (54/P190-B) (CBMAB-P0070-YC)

-
Mouse Anti-ESR1 Recombinant Antibody (Y31) (CBMAB-1208-YC)

-
Mouse Anti-ALOX5 Recombinant Antibody (33) (CBMAB-1890CQ)

-
Mouse Anti-CDKL5 Recombinant Antibody (CBFYC-1629) (CBMAB-C1689-FY)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-ACKR3 Recombinant Antibody (V2-261265) (CBMAB-C1023-LY)

-
Mouse Anti-DLL4 Recombinant Antibody (D1090) (CBMAB-D1090-YC)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-ADGRL2 Recombinant Antibody (V2-58519) (CBMAB-L0166-YJ)

-
Rat Anti-ADAM10 Recombinant Antibody (V2-179741) (CBMAB-A1103-YC)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-1728) (CBMAB-2077-YY)

-
Mouse Anti-C1QC Recombinant Antibody (CBFYC-0600) (CBMAB-C0654-FY)

-
Mouse Anti-CD24 Recombinant Antibody (ALB9) (CBMAB-0176CQ)

-
Mouse Anti-ATM Recombinant Antibody (2C1) (CBMAB-A3970-YC)

-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-BrdU Recombinant Antibody (IIB5) (CBMAB-1038CQ)

-
Rabbit Anti-CCL5 Recombinant Antibody (R0437) (CBMAB-R0437-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








