MTAP Antibodies
Background
MTAP gene encoding a key of purine metabolism enzyme, is mainly responsible for remedial synthesis way of adenosine and methionine. This gene is widely expressed in various tissues, and its products maintain the intracellular purine metabolic balance and the normal progress of methylation reactions by decomposing methylthioadenosine (MTA). Studies have shown that the deletion of MTAP is closely related to the development of certain malignant tumors, and its genomic location is adjacent to the well-known tumor suppressor gene CDKN2A. Scientists first identified this gene in 1984. Subsequent research found that the loss of its expression could serve as a molecular marker for various cancers. The unique metabolic function and clinical relevance of the MTAP gene have made it an important subject in current cancer targeted therapy and metabolic research, providing a theoretical basis for the development of new anti-cancer strategies.
Structure of MTAP
MTAP (methylthioadenosine phosphorylase) is a metabolic enzyme with a molecular weight of approximately 32 kDa, and its precise molecular weight varies slightly among different species:
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 32.0 | 31.8 | 31.9 | 32.1 |
| Primary Structural Differences | Conservative catalytic domain | Highly homologous, with a small amount of amino acid variation | Similarity to human MTAP > 90% | Slightly different enzymatic kinetic characteristics |
MTAP is composed of 283 amino acids and forms a typical α/β folding structure. Its active center contains a key phosphorylation site responsible for catalyzing the catabolic metabolism of methylthioadenosine (MTA). The crystal structure of this enzyme indicates a highly conserved dimer conformation, in which the ATP-binding pocket and the substrate recognition region jointly maintain the catalytic efficiency. The activity of MTAP depends on the assistance of magnesium ions (Mg²⁺), and the flexible ring region (residues 120-150) in its tertiary structure undergoes conformational changes during substrate binding, ensuring precise regulation of metabolic reactions. The absence of this enzyme is closely related to the occurrence of various cancers, making it an important target for tumor metabolism research.
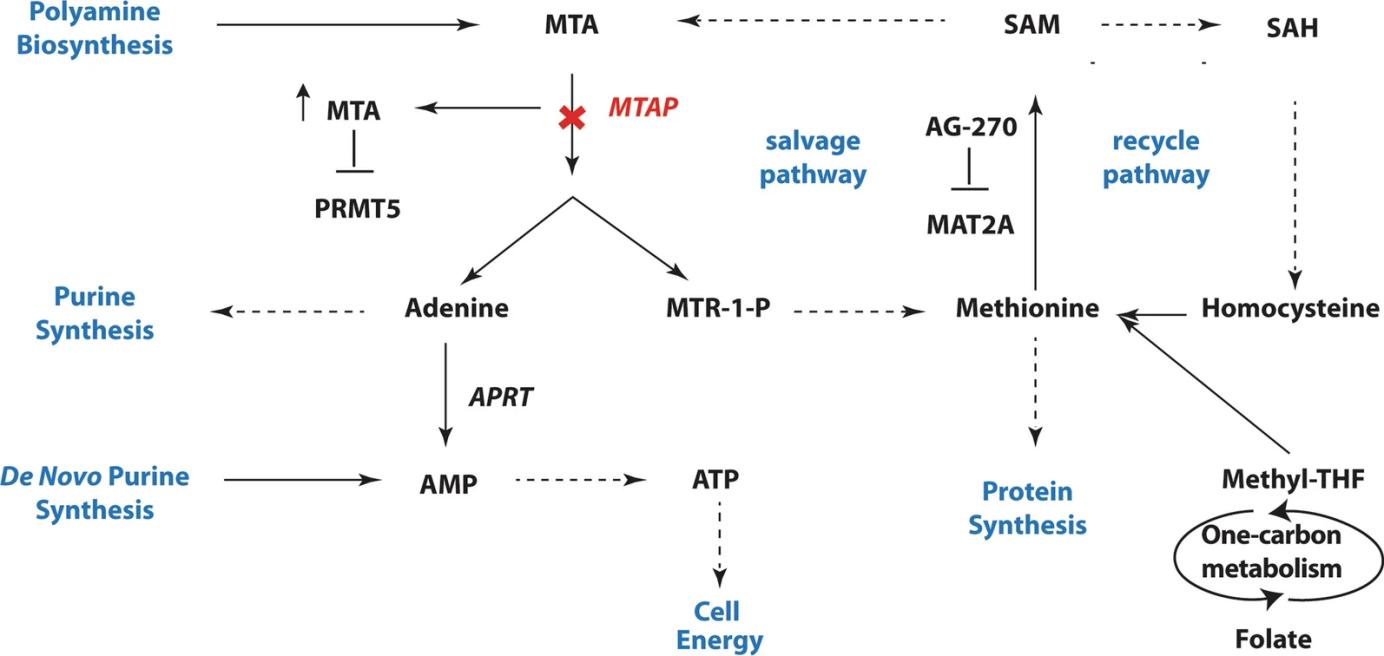 Fig. 1 MTAP metabolism pathway.1
Fig. 1 MTAP metabolism pathway.1
Key structural properties of MTAP:
- Conservative α/β folding structure
- Active center contains key phosphorylation site
- Dimer conformation dependent on magnesium ion (Mg2+)
- Flexible substrate binding ring region (120-150 amino acids)
- Highly conservative ATP-binding pocket
Functions of MTAP
The main function of the MTAP gene is to participate in purine metabolism and methionine rescue pathways, and it also plays an important role in tumor suppression. Its functions can be specifically divided into the following aspects:
| Function | Description |
| Regulation of purine metabolism | Catalyze the decomposition of methylthioadenosine (MTA), maintain the balance of purine nucleotides within cells, and prevent the accumulation of toxic metabolites. |
| Methionine remedial synthesis | By recovering methyl groups from MTA, methionine regeneration is supported, influencing protein methylation and epigenetic regulation. |
| Tumor suppressive effect | The absence of MTAP is associated with various cancers, such as glioma and pancreatic cancer, and its expression can inhibit the proliferation of tumor cells. |
| Chemosensitivity markers | Tumors lacking MTAP may be more sensitive to specific chemotherapy drugs, such as L-alanine analogues. |
| Effects of metabolic reprogramming | By regulating the levels of AMP and methionine, it affects the energy metabolism and methylation modification process of cancer cells. |
The enzymatic activity of MTAP exhibits typical Mie kinetic characteristics, and its substrate specificity is higher than that of ordinary phosphorylases, ensuring the accuracy of metabolic pathways. This gene is widely expressed in normal tissues, but it is often inactivated in some malignant tumors due to the deletion of the 9p21 region, making it a potential biomarker for cancer diagnosis and targeted therapy.
Applications of MTAP and MTAP Antibody in Literature
1. Patro, C. Pawan K., et al. "MTAP loss: a possible therapeutic approach for glioblastoma." Journal of Translational Medicine 20.1 (2022): 620.https://doi.org/10.1186/s12967-022-03823-8
This article reviews the research progress of 5 '-methylthioadenosine phosphorylase (MTAP) gene deletion as a potential therapeutic strategy for glioblastoma, explores its role in tumor metabolism and controversial findings, and points out the research directions that have not yet been explored in this field.
2. Brune, Magdalena M., et al. "MTAP as an emerging biomarker in thoracic malignancies." Lung Cancer 197 (2024): 107963.https://doi.org/10.1016/j.lungcan.2024.107963
This article indicates that MTAP deletion is a novel biomarker for cancers such as NSCLC, associated with 9p21 deletion, and can be detected by immunohistochemistry. Its absence affects the response to immunotherapy and leads to PRMT5-dependence, thus emerging as a new strategy for targeted therapy. This article reviews the role of MTAP in thoracic tumors and its detection methods.
3. Ashok Kumar, Prashanth, et al. "Genomic landscape of non‐small‐cell lung cancer with methylthioadenosine phosphorylase (MTAP) deficiency." Cancer Medicine 12.2 (2023): 1157-1166. https://doi.org/10.1002/cam4.4971
This article indicates that MTAP deletion is observed in 13% of advanced NSCLC cases, associated with a reduction in KRAS mutations, an increase in EGFR mutations, and a lower tumor mutation burden and PD-L1 expression. This absence leads to an increased dependence on PRMT5, providing a new direction for targeted therapy and potentially influencing future combination treatment strategies.
4. Bedard, Gabriel T., et al. "Combined inhibition of MTAP and MAT2a mimics synthetic lethality in tumor models via PRMT5 inhibition." Journal of Biological Chemistry 300.1 (2024).https://doi.org/10.1016/j.jbc.2023.105492
Studies have revealed that the combination of MTAP inhibitor MTDIA and MAT2a inhibitor AG-270 can simulate synthetic lethal effects in MTAP+/+ colorectal cancer cells. By altering the ratio of methylthioadenosine /SAM, it inhibits PRMT5 activity, induces p53 activation and apoptosis, providing a new therapeutic strategy for 98% of non-MTAP deletion CRC cells.
5. Alhalabi, Omar, et al. "MTAP deficiency creates an exploitable target for antifolate therapy in 9p21-loss cancers." Nature communications 13.1 (2022): 1797. https://doi.org/10.1038/s41467-022-29397-z
Studies have revealed that MTAP-deficient tumors are sensitive to pemetrexed treatment. The phase II trial showed that the objective response rate of patients with urothelial carcinoma reached 43%. Preclinical studies have confirmed that the absence of MTAP enhances the efficacy of pemetrexed by interfering with nucleotide metabolism, providing new evidence for synthetic lethal therapy.
Creative Biolabs: MTAP Antibodies for Research
Creative Biolabs specializes in the production of high-quality MTAP antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MTAP Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MTAP antibodies, custom preparations, or technical support, contact us at email.
Reference
- Patro, C. Pawan K., et al. "MTAP loss: a possible therapeutic approach for glioblastoma." Journal of Translational Medicine 20.1 (2022): 620.https://doi.org/10.1186/s12967-022-03823-8
Anti-MTAP antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CTCF Recombinant Antibody (CBFYC-2371) (CBMAB-C2443-FY)

-
Mouse Anti-NSUN6 Recombinant Antibody (D-5) (CBMAB-N3674-WJ)

-
Mouse Anti-CCDC25 Recombinant Antibody (CBLC132-LY) (CBMAB-C9786-LY)

-
Mouse Anti-ABL2 Recombinant Antibody (V2-179121) (CBMAB-A0364-YC)

-
Mouse Anti-AKR1B1 Antibody (V2-2449) (CBMAB-1001CQ)

-
Mouse Anti-CD19 Recombinant Antibody (CBXC-1224) (CBMAB-C1491-CQ)

-
Mouse Anti-BIRC3 Recombinant Antibody (16E63) (CBMAB-C3367-LY)

-
Mouse Anti-CASQ1 Recombinant Antibody (CBFYC-0863) (CBMAB-C0918-FY)

-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

-
Mouse Anti-BZLF1 Recombinant Antibody (BZ.1) (CBMAB-AP705LY)

-
Mouse Anti-AOC3 Recombinant Antibody (CBYY-0014) (CBMAB-0014-YY)

-
Rabbit Anti-ADRA1A Recombinant Antibody (V2-12532) (CBMAB-1022-CN)

-
Mouse Anti-CD33 Recombinant Antibody (6C5/2) (CBMAB-C8126-LY)

-
Mouse Anti-ABIN2 Recombinant Antibody (V2-179106) (CBMAB-A0349-YC)

-
Mouse Anti-CCDC6 Recombinant Antibody (CBXC-0106) (CBMAB-C5397-CQ)

-
Mouse Anti-AZGP1 Recombinant Antibody (CBWJZ-007) (CBMAB-Z0012-WJ)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Mouse Anti-ADIPOR1 Recombinant Antibody (V2-179982) (CBMAB-A1368-YC)

-
Mouse Anti-BLK Recombinant Antibody (CBYY-0618) (CBMAB-0621-YY)

-
Mouse Anti-CEMIP Recombinant Antibody (3C12) (CBMAB-K0296-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








