SPEG Antibodies
Background
The SPEG gene encodes a large serine/threonine protein kinase mainly expressed in myocardial and skeletal muscles. This protein maintains normal muscle contraction and cardiac development by regulating intracellular calcium ion balance and sarcoplasmic reticulum function. Its kinase activity is crucial for protein phosphorylation modification and signal transduction, especially playing a core role in the process of neonatal cardiac maturation. This gene was fully identified in the early 21st century. Research has found that its mutation can cause severe infantile cardiomyopathy or congenital myasthenic syndrome, leading to heart failure and respiratory disorders. As a key component of the myosin pathway, the analysis of the SPEG gene not only reveals a new mechanism of muscle fiber assembly but also provides molecular targets for the diagnosis and treatment of hereditary myopathy, promoting in-depth research on the function of complex kinases.
Structure of SPEG
SPEG is a large serine/threonine protein kinase with a molecular weight of approximately 380 kDa. Its precise molecular weight fluctuates slightly due to transcript differences and post-translational modifications such as phosphorylation.
| Species | Human | Mouse | Zebrafish |
| Molecular Weight (kDa) | 380 | 382 | 375 |
| Primary Structural Differences | Contains 2 kinase domain structure and four domain Ig sample structure | The catalytic activity of the K1 domain is higher | Retain only one kinase domain |
This protein is composed of approximately 3,586 amino acid residues and has a compact conformation composed of multiple domains. Its core region contains two tandem protein kinase domains (K1 and K2), among which the K1 domain has a typical ATP-binding pocket and catalytic ring, while the K2 domain realizes calcium ion perception through calmodulin binding moomers. The N-terminal immunoglobulin-like domain mediates protein-protein interactions, while the conserved aspartic acid residues in the junction region directly participate in substrate recognition. This multi-level structural system jointly ensures its specific functions in myocardial development and calcium homeostasis regulation.
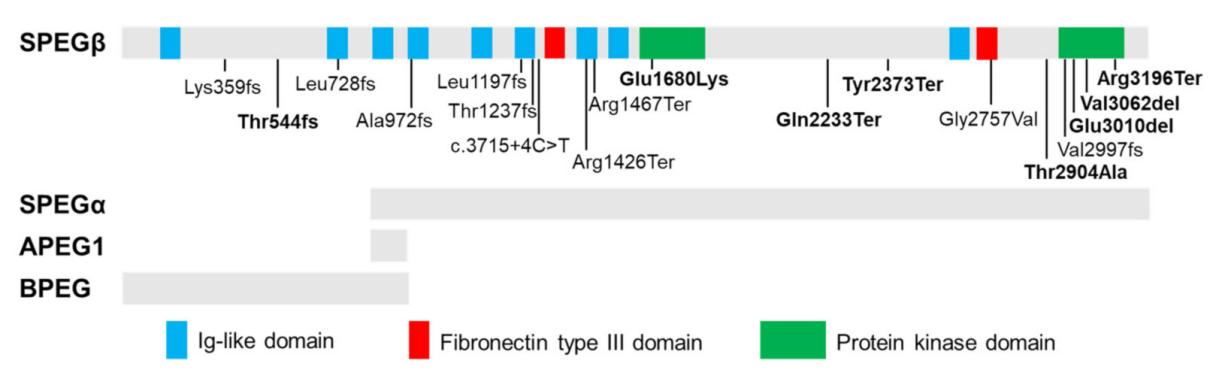 Fig. 1 Tissue-specific isoforms of SPEG and disease-related recessive mutations.1
Fig. 1 Tissue-specific isoforms of SPEG and disease-related recessive mutations.1
Key structural properties of SPEG:
- Tandem arrangement of dual-kinase domains (K1 and K2)
- The N-terminal immunoglobulin-like domain mediates protein interactions
- Calmodulin binding motifs achieve calcium ion-dependent regulation
- Conserved aspartic acid residues are involved in substrate recognition and phosphorylation transfer
Functions of SPEG
The main function of SPEG protein is to regulate calcium homeostasis and signal transduction in myocardial and skeletal muscles. In addition, it is also involved in a variety of physiological and pathological processes, including myocardial maturation, regulation of sarcoplasmic reticulum function, and the occurrence of hereditary myopathy.
| Function | Description |
| Regulation of calcium homeostasis | By phosphorylating key proteins such as RyR2 and SERCA2a, the dynamic balance of calcium ion storage and release within the sarcoplasmic reticulum is precisely regulated. |
| Promotion of myocardial maturation | Drive the structural maturation of the sarcoplasmic reticulum and T-tube system during the neonatal cardiac development stage to ensure the coordination of contractions. |
| Maintenance of contractile function | Ensure the efficiency of the excitation-contraction coupling between myocardial and skeletal muscles and support the metabolic requirements for continuous muscle contraction. |
| Participation of disease mechanisms | The loss of its function leads to autosomal recessive inheritance of infantile dilated cardiomyopathy and myasthenia syndrome. |
| Signal path integration | Through multi-site phosphorylation integration of calcineurin and cross-dialogue with multiple signaling pathways such as CaMKII. |
This protein achieves sequential phosphorylation of different substrates through its unique dual-kinase domain. This multi-level regulatory mechanism explains its core position in the development and function of muscle cells.
Applications of SPEG and SPEG Antibody in Literature
1. Lee, Chang Seok, et al. "Speg interactions that regulate the stability of excitation-contraction coupling protein complexes in triads and dyads." Communications Biology 6.1 (2023): 942. https://doi.org/10.1038/s42003-023-05330-y
This study reveals that in Spegβ -deficient hearts, Spegα can maintain normal cardiac function. Research has found that Spegα can inhibit calcium leakage and protect the transverse tube structure. Meanwhile, Esd, Cmya5 and Fsd2 have been identified as Spegβ -binding proteins to jointly stabilize the excitation-contraction coupling complex. This research provides a new target for the treatment of central nuclear myopathy.
2. Luo, Shiyu, et al. "Striated preferentially expressed protein kinase (SPEG) in muscle development, function, and disease." International journal of molecular sciences 22.11 (2021): 5732. https://doi.org/10.3390/ijms22115732
This study reveals that mutations in the SPEG gene lead to central nuclear myopathy and cardiomyopathy. This review explores its genotype-phenotype relationship and focuses on the multiple functions of SPEG in muscle regeneration, triad structure, and excitation-contraction coupling. Understanding its mechanism of action will provide key therapeutic targets for related diseases.
3. Fleming, Jennifer R., et al. "Exploring obscurin and SPEG kinase biology." Journal of Clinical Medicine 10.5 (2021): 984. https://doi.org/10.3390/jcm10050984
This study focuses on the SPEG kinase domain 1 and its adjacent regions. Unlike obscurin, the interkinase junction region of SPEG is not phosphorylated. Analysis indicates that the SPEG kinase 1 domain possesses catalytic functions and its potential substrates have been identified, providing new clues for revealing its role in muscle.
4. Espinosa, Karla G., et al. "Characterization of a novel zebrafish model of SPEG-related centronuclear myopathy." Disease Models & Mechanisms 15.5 (2022): dmm049437. https://doi.org/10.1242/dmm.049437
In this study, by constructing a SPEG gene double knockout zebrafish model, the key phenotypes of nuclear central myopathy were successfully simulated, including the disruption of the excitation-contraction coupling mechanism and calcium homeostasis disorder. This model reveals disease markers and provides a new platform for in-depth exploration of the pathological mechanisms and therapy evaluation of SPEG-related myopathy.
5. Quan, Chao, et al. "A PKB-SPEG signaling nexus links insulin resistance with diabetic cardiomyopathy by regulating calcium homeostasis." Nature Communications 11.1 (2020): 2186. https://doi.org/10.1038/s41467-020-16116-9
This study reveals that insulin phosphorylates SPEG through PKB, thereby activating its kinase activity and phosphorylating SERCA2a, accelerating the recovery of myocardial calcium ions. The PKB-SPEG-SERCA2a signaling axis is impaired in diabetes, leading to abnormal calcium processing and cardiac dysfunction, which clarifies a new mechanism of diabetic cardiomyopathy.
Creative Biolabs: SPEG Antibodies for Research
Creative Biolabs specializes in the production of high-quality SPEG antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom SPEG Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our SPEG antibodies, custom preparations, or technical support, contact us at email.
Reference
- Luo, Shiyu, et al. "Striated preferentially expressed protein kinase (SPEG) in muscle development, function, and disease." International journal of molecular sciences 22.11 (2021): 5732. https://doi.org/10.3390/ijms22115732
Anti-SPEG antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-BAD (Phospho-Ser136) Recombinant Antibody (CBYY-0138) (CBMAB-0139-YY)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Rabbit Anti-ADRA1A Recombinant Antibody (V2-12532) (CBMAB-1022-CN)

-
Mouse Anti-ENO1 Recombinant Antibody (8G8) (CBMAB-E1329-FY)

-
Mouse Anti-ACTB Recombinant Antibody (V2-179553) (CBMAB-A0870-YC)

-
Mouse Anti-CALR Recombinant Antibody (CBFYC-0763) (CBMAB-C0818-FY)

-
Mouse Anti-ASB9 Recombinant Antibody (1D8) (CBMAB-A0529-LY)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-ADGRE2 Recombinant Antibody (V2-261270) (CBMAB-C0813-LY)

-
Mouse Anti-BACE1 Recombinant Antibody (61-3E7) (CBMAB-1183-CN)

-
Rabbit Anti-CCN1 Recombinant Antibody (CBWJC-3580) (CBMAB-C4816WJ)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Mouse Anti-APCS Recombinant Antibody (CBYC-A663) (CBMAB-A3054-YC)

-
Mouse Anti-ATP1B1 Recombinant Antibody (E4) (CBMAB-0463-LY)

-
Rabbit Anti-AP2M1 (Phosphorylated T156) Recombinant Antibody (D4F3) (PTM-CBMAB-0610LY)

-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

-
Mouse Anti-BBS2 Recombinant Antibody (CBYY-0253) (CBMAB-0254-YY)

-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

-
Mouse Anti-DHFR Recombinant Antibody (D0821) (CBMAB-D0821-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








