ST13 Antibodies
Background
The protein encoded by the ST13 gene, as an auxiliary chaperone molecule interacting with HSP70, is mainly expressed in the endoplasmic reticulum of cells and participates in the folding, transport and stability maintenance processes of various proteins. This gene affects basic physiological activities such as cellular stress response, proliferation and apoptosis by regulating the correct conformation of key signaling proteins. Studies have shown that ST13 plays a significant role in the protective and repair mechanisms of the digestive tract mucosa, and its abnormal expression is closely related to the occurrence and development of inflammatory bowel disease and colorectal tumors. Since its function was revealed, ST13 has become an important model for studying protein quality control systems and cell homeostasis regulation, providing a key entry point for understanding the role of molecular chaperone networks in diseases.
Structure of ST13
The molecular chaperone protein (ST13, also known as Hip or HSPBP1) encoded by the ST13 gene has a molecular weight of approximately 41 kDa. This value is relatively conserved in different mammals, mainly due to the stability requirements of its functional domain.
| Species | Human | Mouse | Rat | Bovine | Pig |
| Molecular Weight (kDa) | 41.0 | 40.8 | 40.9 | 41.1 | 40.9 |
| Primary Structural Differences | Contains the TPR domain, which mediates the interaction with HSP70 | TPR domains are highly homologous and functionally similar | High sequence similarity participates in the stress response | Structure is conserved and function is key in protein folding | Core partner function and important proteins are basically identical |
The ST13 protein contains approximately 360 amino acids, and its three-dimensional structure is mainly composed of several TPR (tetrapeptide repeat) motifs, which are arranged in a typical helical - turning - helical supersecondary structure, forming a bow-shaped hydrophobic groove. This groove, as its main functional interface, can specifically recognize and bind to the C-terminal EEVD motif of HSP70. This interaction does not directly catalyze protein folding but acts as a key regulatory switch, stabilizing the ADP binding state of HSP70 to control the binding and release cycle of HSP70 and its substrate proteins, thereby influencing the overall quality control network of protein folding, transport and degradation within the cell.
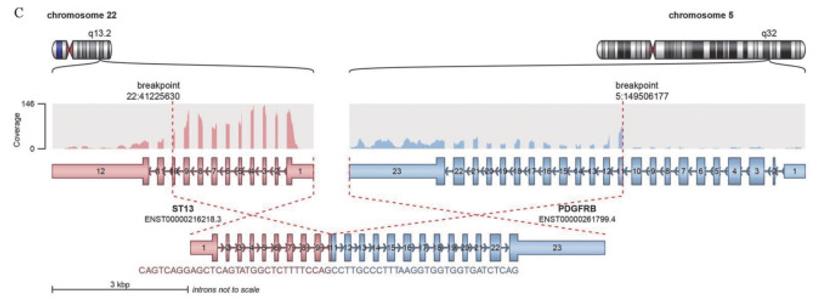 Fig. 1 ST13-PDGFRβ fusion gene identified via whole transcriptome sequencing.1
Fig. 1 ST13-PDGFRβ fusion gene identified via whole transcriptome sequencing.1
Key structural properties of ST13:
- Multiple TPR (tetrapeptide repeat) motifs form a bow-shaped groove structure
- Hydrophobic grooved interface the c-terminal EEVD specificity identification of HSP70 motif
- Do not have catalytic activity, as an allosteric regulation switch control function of HSP70
- By steady state affect the substrate protein HSP70- ADP complex combination and release cycle
Functions of ST13
The protein encoded by the ST13 gene (ST13/HSPBP1) mainly functions as a specific auxiliary regulatory factor for the molecular chaperone HSP70 and plays a key role in various cellular physiological processes.
| Function | Description |
| HSP70 function regulation | By binding to the C-terminal EEVD motif of HSP70, stabilizing its ADP binding state and allosterically regulating the substrate binding and release cycle of HSP70, it serves as the key "timer" or "switch" for the function of HSP70. |
| Protein quality control | By regulating HSP70, it participates in the correct folding of newly synthesized proteins, the refolding of misfolded proteins, and targeted degradation, maintaining intracellular protein homeostasis. |
| Cellular stress response | Under conditions such as heat shock and oxidative stress, changes in its expression or activity can regulate the HSP70-mediated stress protection network and affect cell survival. |
| Signal transduction regulation | By influencing the folding and stability of key signaling proteins (such as kinases and transcription factors), it indirectly participates in regulating signaling pathways such as cell proliferation, differentiation and apoptosis. |
| Disease-related functions | Outstanding role in intestinal mucosal protection and restoration, its expression disorders and inflammatory bowel disease (IBD) and closely related to the occurrence of colorectal cancer development, may affect the stress resistance of tumor cells. |
Unlike the extensive "main force" companion function of HSP70 itself, the role of ST13 is more specific and indirect: it does not directly contact or fold the substrate protein, but acts as a precise allosteric regulator, regulating the functional rhythm of HSP70 by changing its conformational state, thereby precisely coordinating the operation of the entire protein quality control network on a time scale.
Applications of ST13 and ST13 Antibody in Literature
- Wang, Lin-Bo, et al. "Expression of ST13 in colorectal cancer and adjacent normal tissues." World journal of gastroenterology 11.3 (2005): 336. https://dx.doi.org/10.3748/wjg.v11.i3.336
In this article, RNA in situ hybridization technology was used to detect seven pairs of colorectal cancer and adjacent tissues. It was found that the tumor suppressor gene ST13 was generally expressed in adjacent mucosal cells, but its expression was significantly decreased in cancer tissues, suggesting that the down-regulation of ST13 expression may be related to the occurrence of colorectal cancer.
- Cao, Rong-chang, et al. "St13 protects against disordered acinar cell arachidonic acid pathway in chronic pancreatitis." Journal of Translational Medicine 20.1 (2022): 218. https://doi.org/10.1186/s12967-022-03413-8
This study reveals that the chaperone protein St13 stabilizes the IRE1α-XBP1s pathway by binding to Sdf2l1, thereby regulating the arachidonic acid metabolic pathway and alleviating the fat replacement and fibrosis process in chronic pancreatitis, providing a new molecular target for CP treatment.
- Zhou, Xiumei, et al. "Potent and specific antitumor effect for colorectal cancer by CEA and Rb double regulated oncolytic adenovirus harboring ST13 gene." PLOS One (2012): e47566. https://doi.org/10.1371/journal.pone.0047566
In this study, the tumor suppressor gene ST13 was inserted into the oncolytic virus Ad·CEA·E1A(Δ24) to construct a CTGVT-CRC system targeting colorectal cancer. This therapy significantly inhibits tumor growth by activating the P38 MAPK pathway and the mitochondrial apoptotic pathway, and has been granted a Chinese patent.
- Qiu, H. R., et al. "ST13-PDGFRβ positive acute myeloid leukaemia: a case report and literature review." Zhonghua Xueyexue Zazhi 44.8 (2023): 676-679. https://doi.org/10.3760/cma.j.issn.0253-2727.2023.08.011
This study reports the world's first case of acute myeloid leukemia with a positive ST13-PDGFRβ fusion gene. This fusion belongs to the PDGFRβ rearrange-driven disease, providing a new basis for targeted therapy with tyrosine kinase inhibitors.
- Juszkiewicz, Szymon, Sew-Yeu Peak-Chew, and Ramanujan S. Hegde. "Mechanism of chaperone recruitment and retention on mitochondrial precursors." Molecular Biology of the Cell 36.4 (2025): ar39. https://doi.org/10.1091/mbc.E25-01-0035
The article indicates that the mitochondrial targeting sequences (MTS) of mitochondrial precursor proteins regulate the stability of precursor proteins in the cytoplasm and their introduction ability under stress by binding to co-chaperone proteins such as ST13 of Hsc70, in order to maintain mitochondrial homeostasis.
Creative Biolabs: ST13 Antibodies for Research
Creative Biolabs specializes in the production of high-quality ST13 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ST13 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ST13 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Qiu, H. R., et al. "ST13-PDGFRβ positive acute myeloid leukaemia: a case report and literature review." Zhonghua Xueyexue Zazhi 44.8 (2023): 676-679. https://doi.org/10.3760/cma.j.issn.0253-2727.2023.08.011
Anti-ST13 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-4-Hydroxynonenal Recombinant Antibody (V2-502280) (CBMAB-C1055-CN)

-
Mouse Anti-AGO2 Recombinant Antibody (V2-634169) (CBMAB-AP203LY)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Mouse Anti-AKT1/AKT2/AKT3 (Phosphorylated T308, T309, T305) Recombinant Antibody (V2-443454) (PTM-CBMAB-0030YC)

-
Mouse Anti-BZLF1 Recombinant Antibody (BZ.1) (CBMAB-AP705LY)

-
Mouse Anti-COL12A1 Recombinant Antibody (CBYY-C3117) (CBMAB-C4560-YY)

-
Mouse Anti-CAPZB Recombinant Antibody (CBYY-C0944) (CBMAB-C2381-YY)

-
Rat Anti-CD34 Recombinant Antibody (MEC 14.7) (CBMAB-C10196-LY)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Mouse Anti-dsDNA Recombinant Antibody (22) (CBMAB-AP1954LY)

-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

-
Mouse Anti-BANF1 Recombinant Antibody (3F10-4G12) (CBMAB-A0707-LY)

-
Human Anti-SARS-CoV-2 S1 Monoclonal Antibody (CBFYR-0120) (CBMAB-R0120-FY)

-
Mouse Anti-HTLV-1 gp46 Recombinant Antibody (CBMW-H1006) (CBMAB-V208-1154-FY)

-
Rat Anti-CCR2 Recombinant Antibody (475301) (CBMAB-C1338-LY)

-
Mouse Anti-ESR1 Recombinant Antibody (Y31) (CBMAB-1208-YC)

-
Mouse Anti-ALX1 Recombinant Antibody (96k) (CBMAB-C0616-FY)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








