SV2A Antibodies
Background
SV2A protein is a transmembrane glycoprotein existing on the presynaptic membrane of neurons and participates in the regulation of neurotransmitter release as a major component of synaptic vesicles. This protein maintains neurotransmitter homeostasis by mediating the exocytosis cycle and vesicle recirculation process of synaptic vesicles, thereby ensuring normal synaptic transmission in the central nervous system. In the pathogenesis of epilepsy, SV2A has been confirmed as the target of the antiepileptic drug levetiracetam, and its expression level is closely related to the regulation of neuronal excitability. This gene was first identified in the 1990s. The analysis of its three-dimensional structure has promoted the development of targeted drugs for neurological diseases. Continuous research on the structure and function of SV2A not only deepens people's understanding of the mechanism of synaptic plasticity, but also provides important molecular targets for the precise treatment of neuropsychiatric diseases.
Structure of SV2A
SV2A is a transmembrane glycoprotein with a molecular weight of approximately 82 kDa. The actual measured value may fluctuate within the range of approximately 80-100 kDa in the experiment due to the varying degrees of glycosylation modification.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | ~82 | ~82 | ~82 | ~82 |
| Primary Structural Differences | Contains 12 transmembrane domain structure, large area inside the cell | Very high homology with humans | Highly conserved sequence | There are microdifferences between species in the glycosylation sites |
This protein is composed of 727 amino acid residues, and its primary structure folds to form a typical topological structure with 12 transmembrane operations. The tertiary structure of SV2A enclos a central pore within the membrane, and its key functions depend on multiple cytoplasmic domains, which are responsible for interacting with intracellular proteins and regulating vesicle circulation. Specific residues within the transmembrane helix constitute drug binding sites, while N-linked glycosylation modifications are crucial for the correct targeting and stability of proteins.
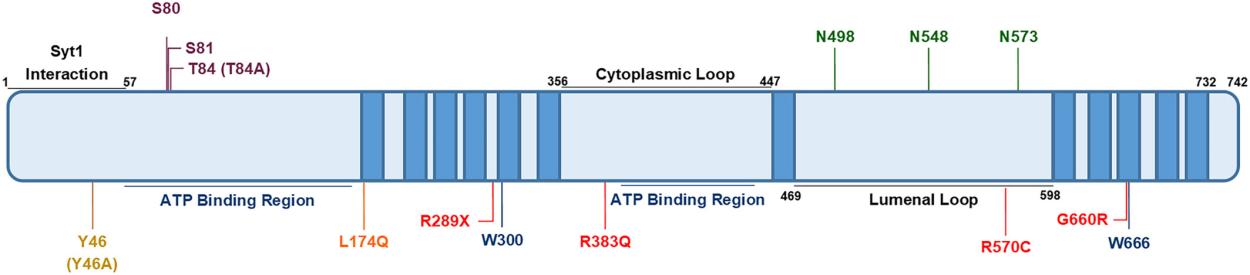 Fig. 1 Key amino acid residues in SV2A.1
Fig. 1 Key amino acid residues in SV2A.1
Key structural properties of SV2A:
- Multiple transmembrane topological structures
- Large hydrophilic cytoplasmic regions
- Specific glycosylation sites
Functions of SV2A
SV2A is a transmembrane glycoprotein with a molecular weight of approximately 82kDa. The actual detected value may fluctuate within the range of approximately 80-100kDa due to differences in the degree of glycosylation modification.
| Function | Description |
| Regulation of vesicle circulation | Participate in the reformation and recycling of synaptic vesicles and maintain the stability of the presynaptic membrane vesicle pool. |
| Neurotransmitter release | Regulating the fusion process of synaptic vesicles and presynaptic membranes directly affects the release efficiency of neurotransmitters. |
| Epilepsy tolerance | As the main target of the antiepileptic drug levetiracetam, its expression level is related to epileptic sensitivity. |
| Vesicle proton gradient maintenance | Assists in maintaining proton gradients within synaptic vesicles, providing the driving force for vesicle uptake of neurotransmitters. |
| Learning and memory support | By ensuring the stability of synaptic transmission under high-frequency stimulation, it participates in neural plasticity processes such as long-term enhancement. |
The functional loss of SV2A can lead to synaptic vesicle circulation disorders and abnormal neurotransmitter release, which is closely related to the pathogenesis of epilepsy and also explains the mechanism of action of antiepileptic drugs targeting it.
Applications of SV2A and SV2A Antibody in Literature
1. Zhang, Yifan, et al. "Connectivity mapping using a novel sv2a loss-of-function zebrafish epilepsy model as a powerful strategy for anti-epileptic drug discovery." Frontiers in Molecular Neuroscience 15 (2022): 881933. https://doi.org/10.3389/fnmol.2022.881933
In this paper, a novel zebrafish sv2a gene knockout model was utilized, and it was found that its deletion would lead to epileptiform discharges and hyperactivity. It was also confirmed that apart from SV2A, levoetrexine may have other antiepileptic targets. The research revealed the related pathogenic pathways through transcriptome analysis and successfully screened out two compounds that can alleviate symptoms by using connectivity maps, providing new ideas for the development of epilepsy drugs.
2. Bertoglio, Daniele, et al. "SV2A PET imaging is a noninvasive marker for the detection of spinal damage in experimental models of spinal cord injury." Journal of nuclear medicine 63.8 (2022): 1245-1251. https://doi.org/10.2967/jnumed.121.263222
Studies have confirmed that SV2A can serve as a novel imaging marker for spinal cord injury. In rodent models, SV2A PET imaging (¹¹C-UCB-J) can non-invasively and sensitively detect significant SV2A deletion in the injury area, and its effect is superior to that of FDG-PET. This technology provides a powerful tool for objectively assessing the degree of spinal cord injury and evaluating new therapies.
3. Yamagata, Atsushi, et al. "Structural basis for antiepileptic drugs and botulinum neurotoxin recognition of SV2A." Nature Communications 15.1 (2024): 3027. https://doi.org/10.1038/s41467-024-47322-4
This study revealed how the full-length SV2A protein binds to botulinum toxin as well as the antiepileptic drugs levetiracetam and buvasetan by analyzing the cryo-electron microscopy structure of the protein. The structure shows that the drug binds to the hypothetical substrate site of SV2A and clarifies the molecular mechanism by which buvasetan has a higher affinity. This discovery provides a key blueprint for the rational design of more effective antiepileptic drugs and neuroimaging tracers.
4. Bavarsad, Mahsa Shanaki, and Lea T. Grinberg. "SV2A PET imaging in human neurodegenerative diseases." Frontiers in Aging Neuroscience 16 (2024): 1380561. https://doi.org/10.3389/fnagi.2024.1380561
This review systematically explores its application in neurodegenerative diseases and compares it with traditional markers such as cognition and imaging. Despite its great potential, the superiority of SV2A as a biomarker still requires more research to confirm, especially by combining longitudinal data with neuropathological verification, in order to fully realize its clinical potential.
5. Hogg, James A., and Michael A. Cousin. "Control of Synaptotagmin‐1 Trafficking by SV2A—Mechanism and Consequences for Presynaptic Function and Dysfunction." Journal of Neurochemistry 169.1 (2025): e16308. https://doi.org/10.1111/jnc.16308
This article reviews the potential core role of SV2A protein in presynaptic function and points out that it may affect neurotransmitter release by regulating the expression and localization of calcium-sensing protein Syt1. The article explores how this interaction explains the pathological mechanism of epilepsy caused by SV2A dysfunction and provides a new perspective on the mechanism of action of related antiepileptic drugs.
Creative Biolabs: SV2A Antibodies for Research
Creative Biolabs specializes in the production of high-quality SV2A antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom SV2A Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our SV2A antibodies, custom preparations, or technical support, contact us at email.
Reference
- Hogg, James A., and Michael A. Cousin. "Control of Synaptotagmin‐1 Trafficking by SV2A—Mechanism and Consequences for Presynaptic Function and Dysfunction." Journal of Neurochemistry 169.1 (2025): e16308. https://doi.org/10.1111/jnc.16308
Anti-SV2A antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

-
Mouse Anti-ARHGAP5 Recombinant Antibody (54/P190-B) (CBMAB-P0070-YC)

-
Rabbit Anti-BAD (Phospho-Ser136) Recombinant Antibody (CAP219) (CBMAB-AP536LY)

-
Mouse Anti-BCL2L1 Recombinant Antibody (H5) (CBMAB-1025CQ)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Mouse Anti-FOXL1 Recombinant Antibody (CBXF-0845) (CBMAB-F0462-CQ)

-
Rat Anti-4-1BB Recombinant Antibody (V2-1558) (CBMAB-0953-LY)

-
Mouse Anti-BCL6 Recombinant Antibody (CBYY-0442) (CBMAB-0445-YY)

-
Mouse Anti-B2M Recombinant Antibody (CBYY-0050) (CBMAB-0050-YY)

-
Mouse Anti-ADRB2 Recombinant Antibody (V2-180026) (CBMAB-A1420-YC)

-
Mouse Anti-8-oxoguanine Recombinant Antibody (V2-7719) (CBMAB-1898CQ)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503417) (CBMAB-V208-1369-FY)

-
Mouse Anti-AKT1 Recombinant Antibody (V2-180546) (CBMAB-A2070-YC)

-
Mouse Anti-BACE1 Recombinant Antibody (CBLNB-121) (CBMAB-1180-CN)

-
Mouse Anti-BAX Recombinant Antibody (CBYY-0216) (CBMAB-0217-YY)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-BIRC7 Recombinant Antibody (88C570) (CBMAB-L0261-YJ)

-
Mouse Anti-CAT Recombinant Antibody (724810) (CBMAB-C8431-LY)

-
Mouse Anti-ALX1 Recombinant Antibody (96k) (CBMAB-C0616-FY)

-
Mouse Anti-CCS Recombinant Antibody (CBFYC-1093) (CBMAB-C1150-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








