Tyrosine hydroxylase Antibodies
Background
Tyrosine hydroxylase is an iron-containing monooxygenase, mainly distributed in the central nervous system and adrenal medulla of mammals. As the rate-limiting enzyme in the biosynthesis of catecholamine neurotransmitters such as dopamine, norepinephrine and epinephrine, this enzyme catalyzes the key step of converting tyrosine to L-dopa, thereby regulating the synthesis level of neurotransmitters. Diving mammals rely on tyrosine hydroxylase to maintain neurotransmitter balance in order to adapt to hypoxic environments. This enzyme was first isolated and identified by the team of Toshiharu Nagatsu in 1964. Its crystal structure was analyzed by X-ray diffraction technology in the 1990s, providing an important template for understanding the mechanism of action of monooxygenase. The unique tetramer structure of tyrosine hydroxylase and its dependence on tetrahydrobiopterin cofactors make it an important target for the study of neurodegenerative diseases and drug development, and it has special value in the research of the pathogenesis of neurological diseases such as Parkinson's disease.
Structure of Tyrosine hydroxylase
Tyrosine hydroxylase is a key enzyme with a molecular weight of approximately 60 kDa, and its precise molecular weight varies slightly among different species (see the table below). This enzyme is composed of a tetramer structure of four identical subunits, each of which contains approximately 500 amino acid residues.
| Species | Human | Rats | Mice | Bovine |
| Molecular Weight (kDa) | 60.2 | 59.8 | 59.9 | 60.5 |
| Primary Structural Differences | Contains an N-terminal regulatory domain | Catalytic core highly conservative | Phosphorylation sites are slightly different | Species-specific variation was found in the C-terminal sequence |
The tertiary structure of TH exhibits a typical α/β folding pattern. Its active center contains non-heme iron ions and works in synergy with the tetrahydrobiopterin (BH4) cofactor. The iron ions in the enzyme molecule are bound through histidine residue coordination, while the conserved phenylalanine triad (Ph-X-Gly -X-Arg-X-Ser) forms the substrate binding pocket. The N-terminal domain contains multiple serine sites that can be phosphorylated by protein kinases (such as Ser19, Ser31, and Ser40), and these modifications can significantly regulate enzyme activity. The stability of the quaternary structure of TH depends on the hydrophobic interaction between subunits, and its active form is a homologous tetramer.
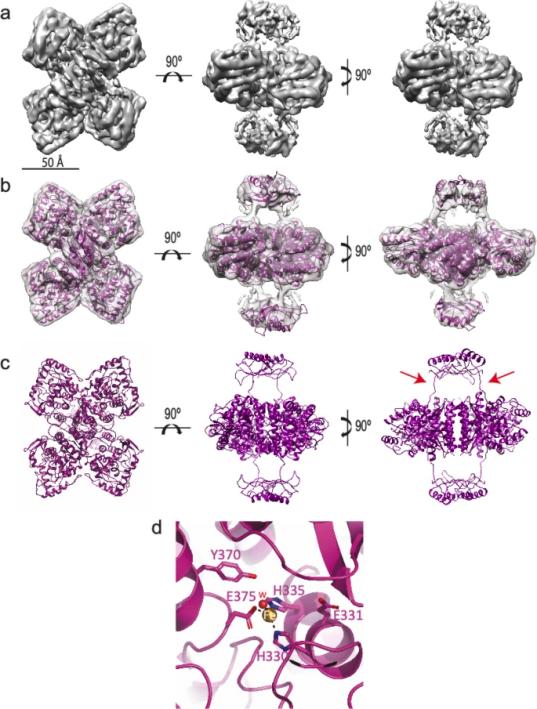 Fig. 1 Structure of the human tyrosine hydroxylase (apo-TH).1
Fig. 1 Structure of the human tyrosine hydroxylase (apo-TH).1
Key structural properties of myoglobin:
- Typical tetrameric α/β fold structure
- Iron-containing active center and tetrahydrobiopterin (BH4) binding site
- Conservative catalytic core domain and controllable N-terminal domain
Functions of Tyrosine hydroxylase
The core function of tyrosine hydroxylase is to catalyze the rate-limiting step in the biosynthesis of catecholamines, while also participating in multiple neuroregulatory processes.
| Function | Description |
| Rate-limiting step in catecholamine synthesis | Catalyzes the conversion of L-tyrosine to L-dopa, determining the rate of dopamine, norepinephrine, and epinephrine synthesis. |
| Regulation of neurotransmitter homeostasis | Precisely regulate the concentration of neurotransmitters in the synaptic cleft through changes in enzyme activity. |
| Regulation of stress response | Under stress conditions, it promotes the rapid synthesis of catecholamines through phosphorylation activation. |
| Developmental neural regulation | Guide the development and differentiation of catecholaminergic neurons during the embryonic period. |
| Oxidative stress response | Its activity is regulated by reactive oxygen species and participates in the pathological process of neurodegenerative diseases. |
The enzymatic kinetics curve of TH exhibits typical characteristics of the Michelin equation, with a Km value of approximately 50-100μM (for tyrosine substrates), and is significantly regulated by the concentration of tetrahydrobiopterin (BH4) and the phosphorylation status. This unique regulatory characteristic makes it an important target for the treatment of neuropsychiatric diseases, especially playing a key role in the treatment strategies for Parkinson's disease.
Applications of Tyrosine hydroxylase and Tyrosine hydroxylase Antibody in Literature
1. Bueno-Carrasco, María Teresa, et al. "Structural mechanism for tyrosine hydroxylase inhibition by dopamine and reactivation by Ser40 phosphorylation." Nature communications 13.1 (2022): 74. https://doi.org/10.1038/s41467-021-27657-y
The article indicates that tyrosine hydroxylase (TH) is a key rate-limiting enzyme in the synthesis of dopamine, and its activity is inhibited by dopamine and activated by phosphorylation of S40. Cryo-electron microscopy structures revealed that dopamine binding fixed the N-terminal helix of TH to the active site, while S40 phosphorylation released the inhibition, revealing the molecular mechanism by which TH regulates dopamine homeostasis.
2. Pomerantz, Seymour H. "The tyrosine hydroxylase activity of mammalian tyrosinase." Journal of Biological Chemistry 241.1 (1966): 161-168. https://doi.org/10.1016/S0021-9258(18)96973-5
Research has found that when tyrosine hydroxylase (TH) catalyzes tyrosine to generate dopa, dopa, as an efficient hydrogen donor, can eliminate reaction delay, but at high concentrations, it inhibits hydroxylation. Tyrosine also shows substrate inhibition at higher concentrations, while ascorbic acid enhances the inhibitory effect of dopa. Compounds such as cyanide also have inhibitory effects on TH activity.
3. Campbell, David G., D. G. Hardie, and P. R. Vulliet. "Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase." Journal of Biological Chemistry 261.23 (1986): 10489-10492. https://doi.org/10.1016/S0021-9258(18)67410-1
Studies have found that tyrosine hydroxylase (TH) in rat pheochromocytoma can be phosphorylated by different protein kinases at multiple serine sites (Ser-8, Ser-19, Ser-40 and Ser-153), all of which are located in the N-terminal region. Phosphorylation modification is of great significance for the regulation of TH activity and catecholamine synthesis.
4. Haycock, John W. "Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40." Journal of Biological Chemistry 265.20 (1990): 11682-11691. https://www.jbc.org/article/S0021-9258(19)38451-0/fulltext
Studies have shown that tyrosine hydroxylase (TH) in PC12 cells is phosphorylated at sites Ser8, Ser19, Ser31 and Ser40. Calcium influx enhances Ser19 phosphorylation, nerve growth factor activates Ser31, cAMP regulates Ser40, while Ser8 phosphorylation is only induced by okada acid. These sites jointly regulate TH activity.
5. Ramsey, Andrew J., Patrick J. Hillas, and Paul F. Fitzpatrick. "Characterization of the active site iron in tyrosine hydroxylase: Redox states of the iron." Journal of Biological Chemistry 271.40 (1996): 24395-24400. https://doi.org/10.1074/jbc.271.40.24395
Research has confirmed that tyrosine hydroxylase (TH) is an iron-containing monooxygenase, and its activity depends on the ferrous state. Recombinant rat TH contains 0.5-0.7 iron atoms/subunits. When isolated, iron is trivalent and can be reduced to a divalent active state. During the catalytic process, enzymes exist in the form of ferrous ions, but some of them will be oxidized. Iron ions participate in catalysis by binding to His331/336 and are close to the substrate binding site.
Creative Biolabs: Tyrosine hydroxylase Antibodies for Research
Creative Biolabs specializes in the production of high-quality Tyrosine hydroxylase antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom Tyrosine hydroxylase Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our Tyrosine hydroxylase antibodies, custom preparations, or technical support, contact us at email.
Reference
- Bueno-Carrasco, María Teresa, et al. "Structural mechanism for tyrosine hydroxylase inhibition by dopamine and reactivation by Ser40 phosphorylation." Nature communications 13.1 (2022): 74. https://doi.org/10.1038/s41467-021-27657-y
Anti-Tyrosine hydroxylase antibodies
 Loading...
Loading...
Hot products 
-
Rat Anti-C5AR1 Recombinant Antibody (8D6) (CBMAB-C9139-LY)

-
Rabbit Anti-B2M Recombinant Antibody (CBYY-0059) (CBMAB-0059-YY)

-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Rat Anti-ADAM10 Recombinant Antibody (V2-179741) (CBMAB-A1103-YC)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Mouse Anti-DMD Recombinant Antibody (D1190) (CBMAB-D1190-YC)

-
Rat Anti-CD34 Recombinant Antibody (MEC 14.7) (CBMAB-C10196-LY)

-
Mouse Anti-CD33 Recombinant Antibody (6C5/2) (CBMAB-C8126-LY)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

-
Mouse Anti-AGO2 Recombinant Antibody (V2-634169) (CBMAB-AP203LY)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-ACLY Recombinant Antibody (V2-179314) (CBMAB-A0610-YC)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-ELAVL4 Recombinant Antibody (6B9) (CBMAB-1132-YC)

-
Rabbit Anti-AP2M1 (Phosphorylated T156) Recombinant Antibody (D4F3) (PTM-CBMAB-0610LY)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Mouse Anti-ATP1A2 Recombinant Antibody (M7-PB-E9) (CBMAB-A4013-YC)

-
Mouse Anti-ABL2 Recombinant Antibody (V2-179121) (CBMAB-A0364-YC)

-
Mouse Anti-AMIGO2 Recombinant Antibody (CBYY-C0756) (CBMAB-C2192-YY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








