ZFX Antibodies
Background
ZFX gene is located on the X chromosome and encodes a zinc finger structure transcription factor that is widely involved in embryonic development and stem cell pluripotency maintenance. This protein regulates the expression of downstream genes by specifically binding to DNA sequences and plays a key role in the sex determination pathway and cell proliferation and differentiation. Since its discovery in 1987, ZFX has become an important research subject in developmental biology and genetics due to its characteristics of evolutionary conservation and functional complexity. Its mutations are associated with a variety of developmental disorders and continue to provide an important paradigm for humans to understand gene regulatory networks and gender-related genetic mechanisms.
Structure of ZFX
The ZFX gene encodes a zinc finger protein with a molecular weight of approximately 58 kDa, a value that is relatively conserved in different mammals. The following table lists the molecular weight and main structural differences of ZFX proteins among different species:
| Species | Human | Mouse | Bovine | Chicken |
| Molecular Weight (kDa) | 58.0 | 57.8 | 58.2 | 57.5 |
| Primary Structural Differences | Type contains 19 C2H2 zinc finger domain structure, rich in acidic amino acid N end | Zinc finger repeat sequence number consistent with human height, high sequence homology | Individual amino acid substitutions are present in the central zinc finger region | Zinc fingers are few in number and the C-terminal sequence is species-specific |
The ZFX protein is composed of at least 754 amino acids, and its structure is mainly made up of tandem C2H2 zinc finger modules. Each zinc finger unit recognizes a specific DNA sequence through ββα folding. The N-terminal of a protein contains a transcriptional activation domain, while the C-terminal is involved in nuclear localization and protein-protein interactions. The cysteine and histidine residues in the zinc finger region coordinate with zinc ions, maintaining spatial conformational stability and thereby achieving specific binding to the promoter region of the target gene.
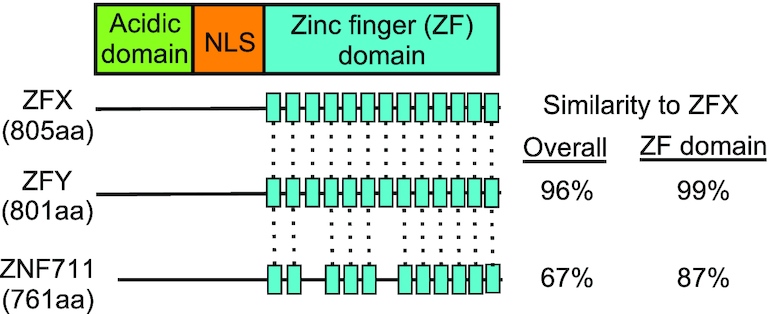 Fig. 1 The ZFX gene family.1
Fig. 1 The ZFX gene family.1
Key structural properties of ZFX:
- Typical C2H2 type zinc finger array structure
- Each zinc finger is coordinated with zinc ions by conserved cysteine and histidine
- N-terminal transcription regulatory region rich in acidic amino acids
- C end contains a signal of check and ratify and protein interaction module
Functions of ZFX
The main function of the ZFX gene is to act as a transcription factor to participate in the regulation of gene expression, especially playing a core role in embryonic development and the maintenance of stem cell pluripotency. In addition, it also involves important biological processes such as sex determination, cell proliferation and apoptosis.
| Function | Description |
| Gene transcriptional regulation | By specifically binding to the promoter of the target gene through the zinc finger domain, the transcription of downstream genes is activated or inhibited. |
| Regulation of embryonic development | In early embryonic high expression, regulation pluripotency related gene networks, affect embryonic stem cell self-renewal and differentiation. |
| Gender determines pathway participation | Located on the X chromosome, it is involved in the fine regulation of mammalian sex determination signaling pathways. |
| Cell cycle and apoptosis influence | Genes that can affect cell proliferation, cell cycle progression and apoptosis play an important role in maintaining tissue homeostasis. |
| Maintenance of stem cell pluripotency | Expressed in various stem cells, it helps maintain an undifferentiated state and regulate key developmental signaling pathways. |
The ZFX protein achieves DNA binding specificity through multiple zinc finger domains. Its regulation is dose-sensitive and interacts with various cofactors to form complexes for precise control of gene expression. The loss of function of this gene can lead to abnormal embryonic development and disorders of stem cell differentiation.
Applications of ZFX and ZFX Antibody in Literature
1. Pourkeramati, Fatemeh, et al. "Differential expression profile of zfx variants discriminates breast cancer subtypes." Iranian biomedical journal 23.1 (2019): 47. https://doi.org/10.29252/.23.1.47
Studies have found that different splicing variants of ZFX have varying expressions in breast cancer tissues: variant 1/3 is highly expressed in cancer tissues, while variant 5 is lowly expressed. Variant 4 is associated with high grade and lymphatic invasion, and its expression is significantly linked to HER2 status, which may serve as a novel molecular marker for the diagnosis and prognosis of breast cancer.
2. Zhang, uyan, et al. "A conserved ZFX/WNT3 axis modulates the growth and imatinib response of chronic myeloid leukemia stem/progenitor cells." Cellular & Molecular Biology Letters 28.1 (2023): 83. https://doi.org/10.1186/s11658-023-00496-z
Research has found that ZFX is highly expressed in CD34+ cells of chronic myeloid leukemia (CML), promoting cell proliferation and inducing imatinib resistance by activating the WNT3/β-catenin signaling pathway. This axis can serve as a potential therapeutic target.
3. Soong, Chen-Pang, and Andrew Arnold. "Recurrent ZFX mutations in human sporadic parathyroid adenomas." Oncoscience 1.5 (2014): 360. https://doi.org/10.18632/oncoscience.116
Research has found that there are high-frequency somatic mutations of the ZFX gene in sporadic parathyroid adenomas, with hotspots located in the highly conserved zinc finger domain (R786/787), suggesting that ZFX may be involved in tumorigenesis as a new proto-oncogene.
4. Malcher, Agnieszka, et al. "Whole‐genome sequencing identifies new candidate genes for nonobstructive azoospermia." Andrology 10.8 (2022): 1605-1624. https://doi.org/10.1111/andr.13269
This study identified multiple potential pathogenic gene variations, including ZFX, in patients with non-obstructive azoospermia (NOA) through whole-genome sequencing, providing new clues for exploring the genetic mechanism of NOA.
5. Rhie, Suhn Kyong, et al. "ZFX acts as a transcriptional activator in multiple types of human tumors by binding downstream from transcription start sites at the majority of CpG island promoters." Genome research 28.3 (2018): 310-320. https://doi.org/10.1101/gr.228809.117
The article indicates that ZFX is highly expressed in various cancers. By binding to a specific position downstream of the CpG island promoter, it activates gene transcription and affects chromatin structure, promoting tumorigenesis and development.
Creative Biolabs: ZFX Antibodies for Research
Creative Biolabs specializes in the production of high-quality ZFX antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ZFX Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ZFX antibodies, custom preparations, or technical support, contact us at email.
Reference
- Ni, Weiya, et al. "Characterization of the ZFX family of transcription factors that bind downstream of the start site of CpG island promoters." Nucleic acids research 48.11 (2020): 5986-6000. https://doi.org/10.1093/nar/gkaa384
Anti-ZFX antibodies
 Loading...
Loading...
Hot products 
-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-ALDOA Recombinant Antibody (A2) (CBMAB-A2316-YC)

-
Mouse Anti-CGAS Recombinant Antibody (CBFYM-0995) (CBMAB-M1146-FY)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Mouse Anti-ACO2 Recombinant Antibody (V2-179329) (CBMAB-A0627-YC)

-
Mouse Anti-ADGRE5 Recombinant Antibody (V2-360335) (CBMAB-C2088-CQ)

-
Mouse Anti-ALOX5 Recombinant Antibody (33) (CBMAB-1890CQ)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Mouse Anti-4-Hydroxynonenal Recombinant Antibody (V2-502280) (CBMAB-C1055-CN)

-
Mouse Anti-BIRC3 Recombinant Antibody (315304) (CBMAB-1214-CN)

-
Mouse Anti-ATP5F1A Recombinant Antibody (51) (CBMAB-A4043-YC)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Mouse Anti-BHMT Recombinant Antibody (CBYY-0547) (CBMAB-0550-YY)

-
Mouse Anti-AOC3 Recombinant Antibody (CBYY-0014) (CBMAB-0014-YY)

-
Mouse Anti-CD46 Recombinant Antibody (CBFYC-0076) (CBMAB-C0085-FY)

-
Mouse Anti-FLT1 Recombinant Antibody (11) (CBMAB-V0154-LY)

-
Mouse Anti-C1QC Recombinant Antibody (CBFYC-0600) (CBMAB-C0654-FY)

-
Mouse Anti-ADAM12 Recombinant Antibody (V2-179752) (CBMAB-A1114-YC)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Rabbit Anti-B2M Recombinant Antibody (CBYY-0059) (CBMAB-0059-YY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






