COL1A1 Antibodies
Background
COL1A1 gene encodes the α1 chain of type 1 collagen, which is the main structural protein that constitutes the extracellular matrix of skin, bone, tendon and other tissues. This gene provides tissue strength and support by forming a triple helix structure and plays a key role in wound repair, bone development and the maintenance of tissue homeostasis. Its mutations are closely related to various connective tissue diseases such as osteogenesis imperfecta (also known as "brittle bone disease") and Ellers-Danlos syndrome. COL1A1 was first identified in 1977 and was the first collagen gene to be fully characterized through molecular cloning technology. Its research has greatly advanced the progress in the fields of extracellular matrix biology, fibrogenesis mechanisms, and the molecular basis of hereditary connective tissue diseases.
Structure of COL1A1
Myoglobin is a relatively small protein with a molecular weight of approximately 16.7 kDa. This weight may slightly vary between species due to minor differences in amino acid sequence.
| Species | Human | Mouse | Bovine | Rat |
| Molecular Weight (kDa) | 138 | 139 | 137 | 138 |
| Primary Structural Differences | Rich in glycine and proline repeat sequences | Similar to humans, the repetitive structure is highly conservative | Collagen domains are highly homologous | High sequence similarity with human COL1A1 |
The COL1A1 protein contains approximately 1,464 amino acids and forms a unique triple helix structure. The stability of this structure depends on the periodic arrangement of the Gly-X-Y repeat sequence (where X is often proline and Y is often hydroxyproline). Each peptide chain first folds into a left-handed helix by itself, and then the three peptide chains entangle with each other to form a superhelical structure. This structure maintains stability through intramolecular hydrogen bonds and hydroxylation modifications, providing strong mechanical support for tissues such as tendons, bones and skin.
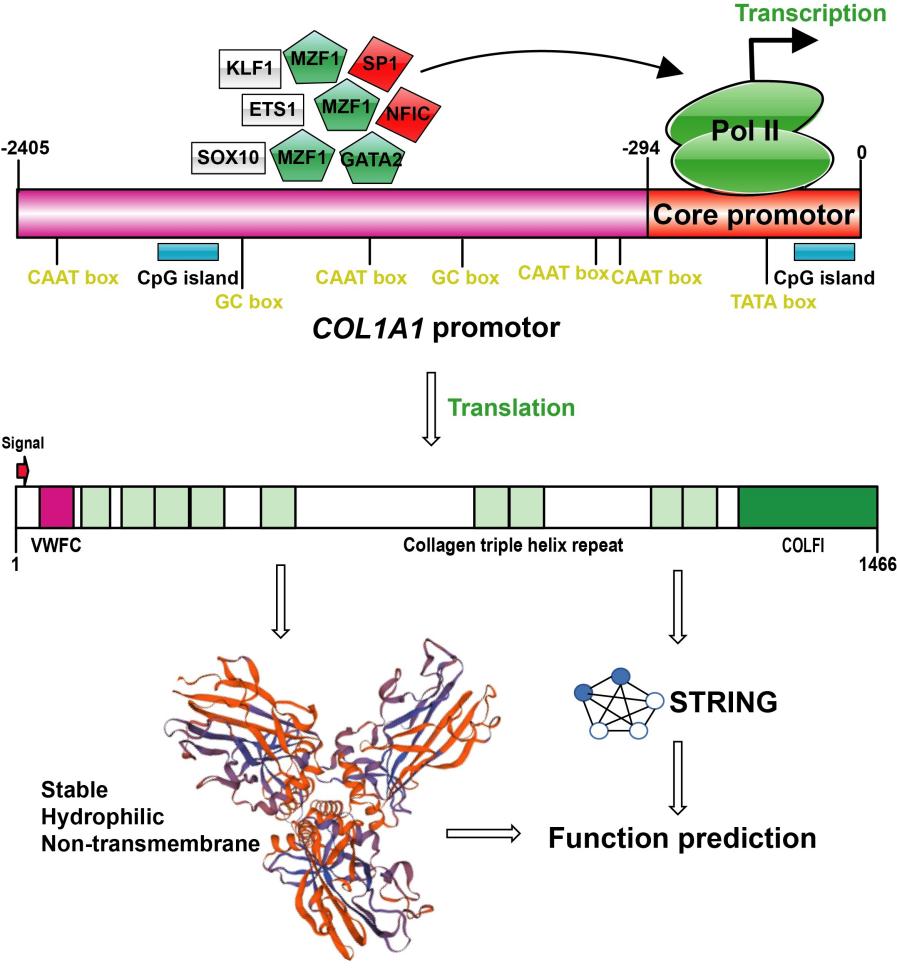 Fig. 1 Promoter structure and protein functional characteristics of COL1A1 gene.1
Fig. 1 Promoter structure and protein functional characteristics of COL1A1 gene.1
Key structural properties of COL1A1:
- Repeat Gly-x-y constitute the triple helical structure of amino acid sequence
- Stable inter-helix hydrogen bond networks and hydroxylation modifications maintain structural integrity
- Amino-terminal and carboxyl-terminal propeptides are involved in the correct assembly and secretion
Functions of COL1A1
The main function of the COL1A1 gene is to constitute type I collagen fibers in the extracellular matrix, providing structural support for connective tissue. In addition, it is also involved in a variety of physiological and pathological processes, including tissue repair, developmental regulation and the occurrence of fibrosis.
| Function | Description |
| Structural support | Form a high-tensile strength collagen fiber network to maintain the integrity and mechanical stability of tissues such as skin, bones, and tendons. |
| Cell adhesion and migration | For cell adhesion matrix, control the movement, proliferation and differentiation of cells. |
| Tissue development and repair | In embryonic development, increased bone healing and wound repair process, promote the generation and remodeling. |
| Regulation of fibrosis | Abnormal expression or metabolic imbalance can lead to fibrosis in organs such as the liver and lungs, and promote excessive deposition of extracellular matrix. |
| Signal transduction participation | By interacting with receptors such as integrins, it affects the activation of intracellular signaling pathways such as TGF-β and MAPK. |
Collagen encoded by COL1A1 forms a triple helical structure. Its thermal stability and protease resistance are much higher than those of many non-fibrin proteins, making it suitable for withstanding long-term mechanical loads and maintaining the stability of tissue structure.
Applications of COL1A1 and COL1A1 Antibody in Literature
1. Ren, Junyu, Junlong Da, and Narisu Hu. "Identification of COL1A1 associated with immune infiltration in brain lower grade glioma." PLoS One 17.7 (2022): e0269533. https://doi.org/10.1371/journal.pone.0269533
This study reveals that COL1A1 is highly expressed in low-grade glioma (LGG), is an independent prognostic factor, and is significantly associated with the infiltration of various immune cells such as CD4+ T cells and CD8+ T cells.
2. Saito, Mika, et al. "Is COL1A1 Gene rs1107946 Polymorphism Associated with Sport Climbing Status and Flexibility?." Genes 13.3 (2022): 403. https://doi.org/10.3390/genes13030403
This study reveals that the AC genotype of the COL1A1 rs1107946 polymorphism is more common among rock climbers and may be related to individual flexibility and the rate of age-related flexibility decline, but the results were not corrected by multiple tests.
3. Pan, Ti-Ti, et al. "A novel mutation in COL1A1 causing osteogenesis imperfecta/hearing loss." Brazilian journal of otorhinolaryngology 89.5 (2023): 101312. https://doi.org/10.1016/j.bjorl.2023.101312
Research has found that the clinical symptoms of OI proband are caused by a heterozygous mutation of c.1922_1923 ins C in the COL1A1 gene, which leads to amino acid frameshift. Stapes surgery can improve the hearing of OI patients.
4. Zhang, Cangang, et al. "COL1A1 is a potential prognostic biomarker and correlated with immune infiltration in mesothelioma." BioMed Research International 2021.1 (2021): 5320941. https://doi.org/10.1155/2021/5320941
Studies have found that COL1A1 is highly expressed in mesothelioma and is associated with a poor prognosis. Its expression is significantly linked to the infiltration of immune cells such as CD4+ T cells and the ECM and PI3K-Akt pathways, and may become a potential biomarker.
5. Zhytnik, Lidiia, et al. "COL1A1/2 pathogenic variants and phenotype characteristics in Ukrainian osteogenesis imperfecta patients." Frontiers in genetics 10 (2019): 722. https://doi.org/10.3389/fgene.2019.00722
A study found that an analysis of 94 Ukrainian osteogenesis imperfections (OI) families revealed that pathogenic variations in the COL1A1 gene accounted for 76.19%, and the severity of the phenotype was significantly correlated with the type of collagen defect. Additionally, 27 new pathogenic variations were discovered.
Creative Biolabs: COL1A1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality COL1A1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom COL1A1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our COL1A1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Gang, Guangming, et al. "Molecular characteristics and promoter analysis of porcine COL1A1." Genes 13.11 (2022): 1971. https://doi.org/10.3390/genes13111971
Anti-COL1A1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AZGP1 Recombinant Antibody (CBWJZ-007) (CBMAB-Z0012-WJ)

-
Mouse Anti-AKT1/AKT2/AKT3 (Phosphorylated T308, T309, T305) Recombinant Antibody (V2-443454) (PTM-CBMAB-0030YC)

-
Mouse Anti-CIITA Recombinant Antibody (CBLC160-LY) (CBMAB-C10987-LY)

-
Mouse Anti-EGR1 Recombinant Antibody (CBWJZ-100) (CBMAB-Z0289-WJ)

-
Mouse Anti-AKT1 Recombinant Antibody (V2-180546) (CBMAB-A2070-YC)

-
Rabbit Anti-B2M Recombinant Antibody (CBYY-0059) (CBMAB-0059-YY)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503417) (CBMAB-V208-1369-FY)

-
Mouse Anti-COL12A1 Recombinant Antibody (CBYY-C3117) (CBMAB-C4560-YY)

-
Mouse Anti-AKT1 (Phosphorylated S473) Recombinant Antibody (V2-505430) (PTM-CBMAB-0067LY)

-
Mouse Anti-DLL4 Recombinant Antibody (D1090) (CBMAB-D1090-YC)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Mouse Anti-ARID3A Antibody (A4) (CBMAB-0128-YC)

-
Mouse Anti-BZLF1 Recombinant Antibody (BZ.1) (CBMAB-AP705LY)

-
Mouse Anti-ATP1A2 Recombinant Antibody (M7-PB-E9) (CBMAB-A4013-YC)

-
Mouse Anti-BIRC7 Recombinant Antibody (88C570) (CBMAB-L0261-YJ)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

-
Rat Anti-EMCN Recombinant Antibody (28) (CBMAB-E0280-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






