DAXX Antibodies
Background
DAXX is a multifunctional nuclear protein that mainly participates in key biological processes such as apoptosis, chromatin remodeling and gene transcriptional regulation. This protein interacts with signaling molecules such as the Fas death receptor through its death domain, thereby activating the apoptotic pathway. At the same time, it functions as a histone molecular chaperone during chromatin assembly. DAXX was first discovered in 1997, and its abnormal expression is closely related to the occurrence and development of various cancers and neurodegenerative diseases. This protein, due to its dual functions in cell fate determination and epigenetic regulation, has become an important object in tumor-targeted therapy and epigenetic research, providing a key perspective for understanding protein-protein interactions and cell signaling networks.
Structure of DAXX
DAXX is a nucleoprotein with a molecular weight of approximately 81.4 kDa. Its precise molecular weight may vary slightly due to differences in species and transcript subtypes.
| Species | Human | Mouse | Rat | Zebrafish |
| Molecular Weight (kDa) | 81.4 | 81.5 | 81.3 | 80.8 |
| Primary Structural Differences | Contains typical death domains and SUMO interaction motifs | High homology with human and highly conserved death domain | Amino acid sequence is slightly different from that of rats | Partial sequence variation exists in the death domain |
This protein is composed of 740 amino acids, and its primary structure includes a C-terminal Death Domain that mediates protein interactions and an N-terminal region rich in acidic amino acids. The secondary structure of DAXX is mainly composed of α -helices, and its dead domain folds into a typical six-helix bundle, which is the key structural basis for its binding to various signaling proteins such as Fas receptor and P53. This domain forms a hydrophobic core through the interaction between helices, providing structural stability for its functional performance.
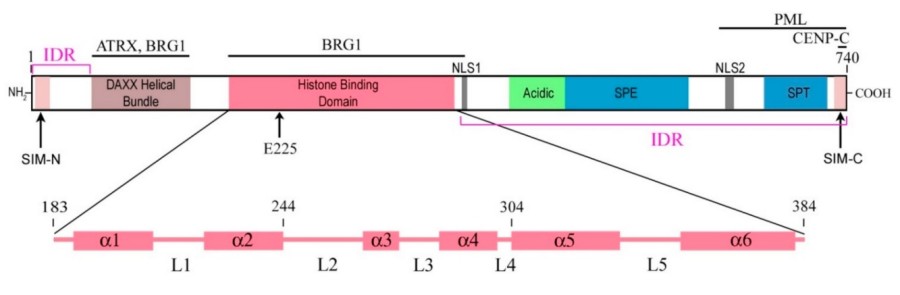 Fig. 1 Simplified diagrams depicting the modular structure of the DAXX molecule.1
Fig. 1 Simplified diagrams depicting the modular structure of the DAXX molecule.1
Key structural properties of DAXX:
- The C-end forms a typical Death Domain, presenting a six-helix bundle conformation
- The N-terminal is an acidic amino acid region rich in serine/proline
- Sumoylation modification sites exist to mediate protein interactions
- Hydrophobic core stable domain folding is formed by helix-helix interactions
Functions of DAXX
The core function of the DAXX protein is to act as a transcriptional regulatory cofactor and a modulator of apoptosis signals. However, it is also widely involved in a variety of key cellular processes, including chromatin remodeling, viral infection response and DNA damage repair.
| Function | Description |
| Transcriptional regulation | As a transcriptional coregulator, it can activate or inhibit the expression of specific genes and participate in the process of cell differentiation and development. |
| Regulation of apoptosis | Through the interaction of the death domain with Fas and other receptors, it is involved in the transduction and regulation of exogenous apoptosis pathway signals. |
| Chromatin remodeling | As a histone molecular chaperone, it promotes the deposition of H3.3 histones in specific regions of chromatin and influences the epigenetic state. |
| DNA damage response | In the process of DNA double-strand break repair, it is recruited to the damage site and involved in the maintenance of genomic stability. |
| Antiviral innate immunity | Inhibit viral replication and regulate the interferon pathway related gene expression, involved in cellular antiviral immune response. |
The interaction mode between DAXX and DNA is multivalent and highly dynamic, which is consistent with its inherent disordered protein characteristics, enabling it to flexibly participate in the cross-regulation of multiple signaling pathways and play a core role in cell fate determination.
Applications of DAXX and DAXX Antibody in Literature
1. Mahmud, Iqbal, and Daiqing Liao. "DAXX in cancer: phenomena, processes, mechanisms and regulation." Nucleic acids research 47.15 (2019): 7734-7752. https://doi.org/10.1093/nar/gkz634
The article indicates that DAXX is a multifunctional protein, and its overexpression is commonly seen in various cancers, promoting tumor occurrence, progression and treatment resistance. It affects transcription, DNA repair and apoptosis through molecular chaperone activity, epigenetic regulation and protein interaction, and its activity is regulated by post-translational modification and phase separation degradation mechanisms.
2. Carraro, Massimo, et al. "DAXX adds a de novo H3. 3K9me3 deposition pathway to the histone chaperone network." Molecular cell 83.7 (2023): 1075-1092. https://doi.org/10.1016/j.molcel.2023.02.009
The article indicates that through exploratory interaction omics, this study reveals a new mechanism by which histone partner DAXX recruits methyltransferase, catalyzes H3K9me3 and drives heterochromatin assembly before histone H3.3-H4 deposition, providing a new framework for understanding the targeted deposition of modified histones and the establishment of chromatin states.
3. Clatterbuck Soper, Sarah F., and Paul S. Meltzer. "ATRX/DAXX: Guarding the Genome against the Hazards of ALT." Genes 14.4 (2023): 790. https://doi.org/10.3390/genes14040790
The article indicates that DAXX forms a histone chaperone complex with ATRX, which is responsible for depositing the histone variant H3.3 and maintaining telomere and heterochromatin stability. Its functional loss is closely related to the AlT-type telomere elongation mechanism, which is more common in specific tumors and promotes genomic instability and tumor development.
4. Bogolyubova, Irina, and Dmitry Bogolyubov. "DAXX is a crucial factor for proper development of mammalian oocytes and early embryos." International Journal of Molecular Sciences 22.3 (2021): 1313. https://doi.org/10.3390/ijms22031313
The article indicates that DAXX is a multifunctional nucleoprotein that deposits histone variant H3.3 through chaperone interaction, regulating chromatin silencing, genomic stability and early embryonic development. Its absence will affect ATRX recruitment, telomere function and repeat sequence transcription, leading to a decrease in embryo survival rate.
5. Wang, Fei, et al. "Prognostic significance of altered ATRX/DAXX gene in pancreatic neuroendocrine tumors: a meta-analysis." Frontiers in endocrinology 12 (2021): 691557. https://doi.org/10.3389/fendo.2021.691557
The article indicates that mutations in the DAXX/ATRX gene are associated with the prognosis of pancreatic neuroendocrine tumors (PanNETs). Meta-analysis shows that mutations can significantly increase the disease-free survival and recurrence risk of patients, but have no significant impact on overall survival. In patients with metastasis, mutations may indicate a trend of improved survival.
Creative Biolabs: DAXX Antibodies for Research
Creative Biolabs specializes in the production of high-quality DAXX antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom DAXX Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our DAXX antibodies, custom preparations, or technical support, contact us at email.
Reference
- Bogolyubova, Irina, and Dmitry Bogolyubov. "DAXX is a crucial factor for proper development of mammalian oocytes and early embryos." International Journal of Molecular Sciences 22.3 (2021): 1313. https://doi.org/10.3390/ijms22031313
Anti-DAXX antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

-
Mouse Anti-EGR1 Recombinant Antibody (CBWJZ-100) (CBMAB-Z0289-WJ)

-
Mouse Anti-APOE Recombinant Antibody (A1) (CBMAB-0078CQ)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

-
Mouse Anti-ADV Recombinant Antibody (V2-503423) (CBMAB-V208-1364-FY)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-CD24 Recombinant Antibody (ALB9) (CBMAB-0176CQ)

-
Rat Anti-ADGRE4 Recombinant Antibody (V2-160163) (CBMAB-F0011-CQ)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Mouse Anti-CHRNA9 Recombinant Antibody (8E4) (CBMAB-C9161-LY)

-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

-
Mouse Anti-BIRC3 Recombinant Antibody (315304) (CBMAB-1214-CN)

-
Mouse Anti-AK4 Recombinant Antibody (V2-180419) (CBMAB-A1891-YC)

-
Mouse Anti-CD63 Recombinant Antibody (CBXC-1200) (CBMAB-C1467-CQ)

-
Mouse Anti-CDKL5 Recombinant Antibody (CBFYC-1629) (CBMAB-C1689-FY)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-CCN2 Recombinant Antibody (CBFYC-2383) (CBMAB-C2456-FY)

-
Mouse Anti-ACO2 Recombinant Antibody (V2-179329) (CBMAB-A0627-YC)

-
Rat Anti-CCR2 Recombinant Antibody (475301) (CBMAB-C1338-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








