Fibronectin Antibodies
Background
Found in the matrix and blood plasma is a glycoprotein known as fibronectin that serves an important function, in cell adhesion and migration as well as tissue repair by interacting with integrins and other components of the extracellular matrix (ECM). This protein aids in cell attachment to surfaces. Plays a role in wound healing and supporting the development of embryos due to its fibrous nature that forms a framework guiding cell movement and tissue structure organization since it was initially documented in the 1970s. Extensive research has been conducted on fibronectin to understand its involvement in processes such, as cancer advancement and immune system responses. Its diverse capabilities position it as a contributor, to upholding the integrity and optimal performance of tissues.
Structure of Fibronectin
Fibronectin is a glycoprotein weighing around 220–250 kDa with its size being changeable because of ways it can be altered and modified after translation into proteins; it exists in the extracellular matrix and plasma where it holds important functions, in cell attachment and movement as well as in healing damaged tissues.
| Type | Cellular Fibronectin | Plasma Fibronectin |
| Molecular Weight (kDa) | 230-275 | 250-270 |
| Location of presence | Mainly found in the extracellular matrix, secreted by fibroblasts, epithelial cells and many other cells | Mainly found in plasma and secreted by hepatocytes |
The protein fibronectin exists as a large glycoprotein which contains multiple domains and maintains an elongated flexible structure. Fibronectin protein structure includes type I, II and III repeating modules which enable diverse binding interactions. The dimeric structure of fibronectin emerges from disulfide bonds that connect its two subunits. Fibronectin contains a secondary structure which combines alpha-helices and beta-sheets to create its modular architecture. The type III domains play a crucial role in both cell adhesion and matrix assembly processes. The protein functions as a cell and extracellular matrix component binder which enables tissue repair and cell migration processes.
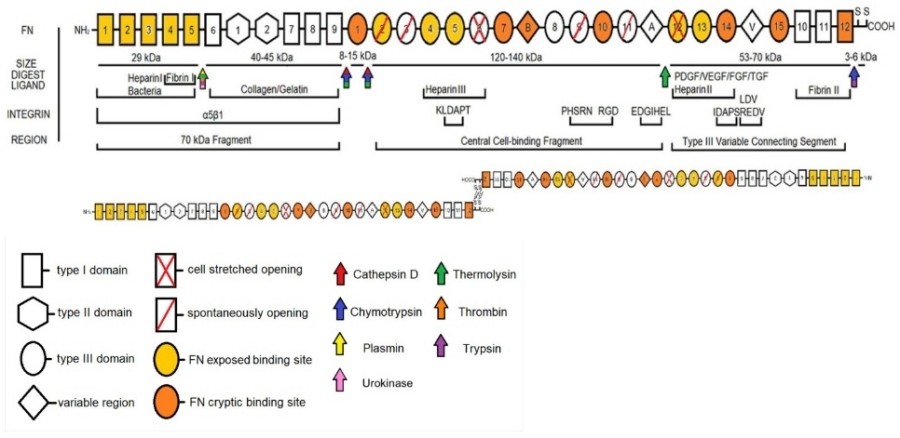 Fig. 1 A schematic of the domains of FN with relevant structural features, cleavage sites, nomenclature, and integrin binding.1
Fig. 1 A schematic of the domains of FN with relevant structural features, cleavage sites, nomenclature, and integrin binding.1
Key structural properties of fibronectin:
- Dimeric structure with disulfide-linked subunits.
- Modular architecture with type I, II, and III domains.
- Type I and II domains for stabilization.
- Type III domains for cell adhesion.
- Flexible, elongated conformation.
- Multiple binding sites for collagen, heparin, and integrins.
Functions of Fibronectin
The main role of fibronectin is to help cells stick together and build the matrix in the body tissues and organs; it also plays a part, in functions like healing wounds and guiding cell movement, throughout the body.
| Function | Description |
| Cell Adhesion | Promotes attachment of cells to the extracellular matrix. |
| Matrix Assembly | Facilitates organization of collagen and other matrix components. |
| Wound Healing | Enhances cell migration and tissue repair at injury sites. |
| Cell Migration | Guides cell movement during development and immune responses. |
| Embryonic Development | Essential for morphogenesis and tissue organization. |
| Regulation of Growth | Influences cell proliferation and differentiation. |
Fibronectins strong bond, with integrins sets it apart from matrix (ECMs) showcasing its essential role, in promoting cell adhesion and movement.
Applications of Fibronectin and Fibronectin Antibody in Literature
1. Yoder, Mervin C. "Therapeutic administration of fibronectin: current uses and potential applications." Clinics in perinatology 18.2 (1991): 325-341. https://doi.org/10.1016/S0095-5108(18)30526-8
This article reviews the therapeutic administration of fibronectin, detailing its current uses in promoting wound healing and tissue repair. It also explores potential applications in regenerative medicine and cancer therapy, highlighting its significance as a multifunctional protein.
2. Patten, Jennifer, and Karin Wang. "Fibronectin in development and wound healing." Advanced drug delivery reviews 170 (2021): 353-368. https://doi.org/10.1016/j.addr.2020.09.005
The article highlights fibronectin as a key factor in embryonic development and wound healing, and discusses its roles in cell adhesion, migration, and tissue repair.
3. Han, Hyeong-jun, et al. "Fibronectin regulates anoikis resistance via cell aggregate formation." Cancer letters 508 (2021): 59-72. https://doi.org/10.1016/j.canlet.2021.03.011
The article highlights fibronectin as a key regulator of anoikis resistance, promoting cell survival via aggregate formation and ECM interactions.
4. Zollinger, Alicia J., and Michael L. Smith. "Fibronectin, the extracellular glue." Matrix Biology 60 (2017): 27-37. https://doi.org/10.1016/j.matbio.2016.07.011
The article emphasizes the importance of fibronectin, as a protein in the matrix that serves as a key element, for cell adhesion and maintaining tissue structure and stability overall.
5. Chernousov, M. A., et al. "Monoclonal antibody to fibronectin which inhibits extracellular matrix assembly." FEBS letters 217.1 (1987): 124-128. https://doi.org/10.1016/0014-5793(87)81255-3
The article discusses an antibody that focuses on fibronectin to prevent the creation of the matrix and highlights its potential, for studying the role of fibronectin and disrupting matrix formation, in disease models.
Creative Biolabs: Fibronectin Antibodies for Research
Creative Biolabs has expertise, in creating top notch fibronectin antibodies for research and medical purposes with a range of monoclonal and polyclonal antibodies designed for ELISA tests Western blots and other diagnostic methods, in our collection.
- Custom Fibronectin Antibody Development: Bespoke solutions to fulfill precise research needs.
- Scalable Production: High-volume antibody manufacturing for industrial partners.
- Expert Consultation: Professional support for optimizing protocols and troubleshooting.
- Aliquot Customization: Practical aliquot sizes for extended storage and reliable results.
For further information regarding our fibronectin antibodies, custom services, or technical assistance, please reach out via info@creative-biolabs.com.
Reference
- Dalton, Caleb J., and Christopher A. Lemmon. "Fibronectin: molecular structure, fibrillar structure and mechanochemical signaling." Cells 10.9 (2021): 2443. https://doi.org/10.3390/cells10092443
Anti-Fibronectin antibodies
Hot products 
-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Armenian hamster Anti-CD40 Recombinant Antibody (HM40-3) (CBMAB-C10365-LY)

-
Mouse Anti-dsDNA Recombinant Antibody (22) (CBMAB-AP1954LY)

-
Mouse Anti-AMOT Recombinant Antibody (CBYC-A564) (CBMAB-A2552-YC)

-
Rabbit Anti-ALOX5AP Recombinant Antibody (CBXF-1219) (CBMAB-F0750-CQ)

-
Mouse Anti-CCS Recombinant Antibody (CBFYC-1093) (CBMAB-C1150-FY)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Mouse Anti-CD46 Recombinant Antibody (CBFYC-0076) (CBMAB-C0085-FY)

-
Rat Anti-ADGRE4 Recombinant Antibody (V2-160163) (CBMAB-F0011-CQ)

-
Mouse Anti-C5b-9 Recombinant Antibody (aE11) (CBMAB-AO138LY)

-
Mouse Anti-CCL18 Recombinant Antibody (64507) (CBMAB-C7910-LY)

-
Rat Anti-EMCN Recombinant Antibody (28) (CBMAB-E0280-FY)

-
Mouse Anti-AP4E1 Recombinant Antibody (32) (CBMAB-A2996-YC)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-APC Recombinant Antibody (CBYC-A661) (CBMAB-A3036-YC)

-
Rabbit Anti-ENO2 Recombinant Antibody (BA0013) (CBMAB-0272CQ)

-
Mouse Anti-CD24 Recombinant Antibody (2Q1282) (CBMAB-C1624-CN)

-
Mouse Anti-ATP1A2 Recombinant Antibody (M7-PB-E9) (CBMAB-A4013-YC)

-
Mouse Anti-BCL6 Recombinant Antibody (CBYY-0442) (CBMAB-0445-YY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot







