GIPR Antibodies
Background
The GIPR gene encodes a G protein-coupled receptor, which is mainly distributed in the pancreas, adipose tissue and the brain, etc. This receptor regulates insulin secretion by specifically binding to gastric inhibitory peptide (GIP), thereby participating in the maintenance of blood glucose homeostasis and having a significant impact on lipid metabolism balance. Due to its core role in energy metabolism, GIPR has become one of the key targets in the development of drugs for type 2 diabetes. Related research not only promotes the development of peptide drugs but also deepens people's understanding of the hormone-receptor interaction mechanism in metabolic diseases.
Structure of GIPR
GIPR is a G protein-coupled receptor with a molecular weight of approximately 44-48 kDa. This value varies among different species, mainly due to the natural differences in the degree of receptor glycosylation modification and extracellular domain structure.
| Species | Human | Mouse | Rat | Pig | Rhesus monkey |
| Molecular Weight (kDa) | 47.5 | 46.8 | 46.9 | 47.2 | 47.4 |
| Primary Structural Differences | Classical GPCR seven-fold transmembrane structure | High homology with human | There are subtle differences in the intracellular loop sequence | Highly conserved ligand binding domain | Commonly used in preclinical studies |
This receptor is composed of 463 amino acids, and its polypeptide chain folds to form a typical sevenfold transmembrane topology. The N-terminal extracellular domain of GIPR is responsible for recognizing and binding to gastric inhibitory peptides (GIP), and this interaction triggers conformational changes in the receptor, thereby activating the downstream Gs protein signaling pathway. The intracellular loop between its transmembrane helix 5 and 6 is crucial for G protein coupling, while the C-terminal domain is involved in receptor regulation and internalization processes.
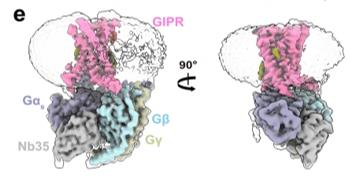 Fig. 1 Cryo-EM density maps of the GIPR–Gs.1
Fig. 1 Cryo-EM density maps of the GIPR–Gs.1
Key structural properties of GIPR:
- Typical seven-fold transmembrane helical structure
- Extracellular n-terminal structural domain is responsible for the specific ligand recognition
- Conservative across membrane area to activate intracellular G protein signaling pathways
Functions of GIPR
The core function of the protein encoded by the GIPR gene is to mediate the hormone signal transduction of gastric inhibitory peptides and participate in the regulation of energy metabolism balance. In addition, this receptor is also involved in physiological processes such as lipid metabolism regulation and neuroprotection.
| Function | Description |
| Regulation of insulin secretion | In response to elevated blood sugar, it promotes the secretion of insulin by pancreatic β cells and enhances the ability to process glucose. |
| Regulation of lipid metabolism | Role in fat cells, affecting lipid storage and decomposition, involved in maintaining the balance of body fat. |
| Influence of bone metabolism | Regulating osteoblast activity through an indirect mechanism has a certain promoting effect on bone density formation. |
| Neuroprotective effect | Activating relevant signaling pathways in the brain may have a supportive effect on the survival and function of neurons. |
| Drug target function | As a key component of dual-target agonists, it is used for the treatment of type 2 diabetes and obesity. |
The dose-effect curve of GIPR against GIP shows typical saturation characteristics. After binding to ligands, it mainly exerts its effect by activating the cAMP-PKA signaling pathway. The functional differences between GIPR in the pancreas and the brain may be related to the tissue-specific signal transduction mechanism.
Applications of GIPR and GIPR Antibody in Literature
1. Veniant, Murielle M., et al. "A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings." Nature Metabolism 6.2 (2024): 290-303. https://doi.org/10.1038/s42255-023-00966-w
The article indicates that the novel therapy AMG 133 targeting GIPR, through the dual mechanism of antagonizing GIPR and activating GLP-1R, has demonstrated significant and long-lasting weight loss effects in clinical trials, with broad prospects.
2. Zhang, Qian, et al. "The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling." Cell metabolism 33.4 (2021): 833-844. https://doi.org/10.1016/j.cmet.2021.01.015
This article demonstrates that myoglobin plays a central role in rhabdomyolysis- and crush syndrome-associated acute kidney injury (RM/CS-AKI), and highlights the potential of a high-affinity anti-myoglobin rabbit monoclonal antibody (RabMAb) as an effective emergency treatment that blocks myoglobin-induced kidney toxicity.
3. El, Kimberley, et al. "The incretin co-agonist tirzepatide requires GIPR for hormone secretion from human islets." Nature Metabolism 5.6 (2023): 945-954. https://doi.org/10.1038/s42255-023-00811-0
This article utilizes mass spectrometry to characterize the binding strength and dissociation dynamics of myoglobin and its antibody-epitope complexes, providing a methodological framework for quantifying protein-ligand interactions and advancing epitope mapping in antibody research.
4. Gutgesell, Robert M., et al. "GIPR agonism and antagonism decrease body weight and food intake via different mechanisms in male mice." Nature metabolism (2025): 1-17. https://doi.org/10.1038/s42255-025-01294-x
Studies have shown that GIPR agitation and antagonism reduce body weight through different mechanisms. Among them, the efficacy of GIPR antagonists completely depends on the functional GLP-1R signal, and its mechanism of action is highly similar to that of the GLP-1R signal.
5. Kizilkaya, Hüsün S., et al. "Characterization of genetic variants of GIPR reveals a contribution of β-arrestin to metabolic phenotypes." Nature metabolism 6.7 (2024): 1268-1281. https://doi.org/10.1038/s42255-024-01061-4
Research reveals that the recruitment of β -inhibitory proteins is crucial for the function of GIPR. It not only affects the integrity of receptor signal transduction, but is also directly associated with a lower obesity-related phenotype, providing a new direction for the development of drugs targeting GIPR.
Creative Biolabs: GIPR Antibodies for Research
Creative Biolabs specializes in the production of high-quality GIPR antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom GIPR Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our GIPR antibodies, custom preparations, or technical support, contact us at email.
Reference
- Cong, Zhaotong, et al. "Molecular features of the ligand-free GLP-1R, GCGR and GIPR in complex with Gs proteins." Cell Discovery 10.1 (2024): 18. https://doi.org/10.1038/s41421-024-00649-0
Anti-GIPR antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Mouse Anti-CCND2 Recombinant Antibody (DCS-3) (CBMAB-G1318-LY)

-
Mouse Anti-AQP2 Recombinant Antibody (G-3) (CBMAB-A3359-YC)

-
Mouse Anti-AP4E1 Recombinant Antibody (32) (CBMAB-A2996-YC)

-
Rabbit Anti-ADRA1A Recombinant Antibody (V2-12532) (CBMAB-1022-CN)

-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

-
Mouse Anti-BAX Recombinant Antibody (CBYY-0216) (CBMAB-0217-YY)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-DDC Recombinant Antibody (8E8) (CBMAB-0992-YC)

-
Mouse Anti-ADIPOR1 Recombinant Antibody (V2-179982) (CBMAB-A1368-YC)

-
Mouse Anti-CAPZB Recombinant Antibody (CBYY-C0944) (CBMAB-C2381-YY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-ACVR1C Recombinant Antibody (V2-179685) (CBMAB-A1041-YC)

-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

-
Mouse Anti-CD63 Recombinant Antibody (CBXC-1200) (CBMAB-C1467-CQ)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Mouse Anti-C4B Recombinant Antibody (CBYY-C2996) (CBMAB-C4439-YY)

-
Mouse Anti-ESR1 Recombinant Antibody (Y31) (CBMAB-1208-YC)

-
Mouse Anti-C5b-9 Recombinant Antibody (aE11) (CBMAB-AO138LY)

-
Mouse Anti-FOXL1 Recombinant Antibody (CBXF-0845) (CBMAB-F0462-CQ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








