MCAM Antibodies
Background
The MCAM gene encodes a single transmembrane glycoprotein, which is mainly expressed on the surface of endothelial cells, melanoma cells and certain stem cells. This protein, as a cell adhesion molecule, participates in processes such as cell migration, proliferation and signal transduction by mediating the interactions between cells or between cells and the matrix. Especially in tumor biology, MCAM has been confirmed to promote the invasion and metastasis of cancer cells such as melanoma, and its overexpression is often associated with a poor prognosis for patients. Since its identification in the early 1990s, MCAM has become an important object of cancer targeted therapy research due to its key role in angiogenesis and tumor progression. The study of its molecular mechanism has deepened our understanding of the function of cell adhesion molecules in disease development.
Structure of MCAM
MCAM is a single-pass transmembrane glycoprotein with a molecular weight of approximately 70-75 kDa, and its precise weight varies depending on the degree of glycosylation modification.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | 70-75 | 68-72 | 69-73 |
| Primary Structural Differences | Containing 647 amino acids, extracellular region has five immunoglobulin domain sample structure | Highly homologous to humans, with subtle differences in amino acids | Structure of domain and human are basically identical |
This protein is composed of 647 amino acids, and its primary structure forms a long extracellular region, a transmembrane region and a short intracellular tail. The secondary structure of MCAM is mainly composed of β -folds, which form the immunoglobulin-like domain (V-V-C2-C2-C2) in its extracellular region, thereby mediating specific adhesion interactions between cells and between cells and the matrix.
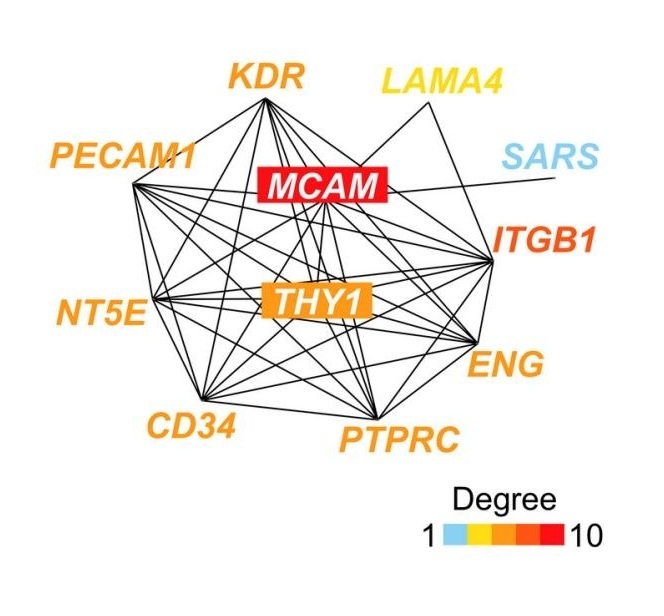 Fig. 1 Network of the top 10 genes correlated with MCAM.1
Fig. 1 Network of the top 10 genes correlated with MCAM.1
Key structural properties of MCAM:
- Extracellular region contains five immunoglobulin sample structure domain (V - V - C2 - C2 - C2)
- The transmembrane single α -helical structure is anchored to the cell membrane
- The shorter intracellular tail is involved in intracellular signal transduction
Functions of MCAM
The main function of the MCAM protein is to mediate cell adhesion and signal transduction. However, it is also widely involved in various pathophysiological processes, especially playing a key role in tumorigenesis and angiogenesis.
| Function | Description |
| Cell adhesion | By binding to ligands such as laminin via its extracellular immunoglobulin-like domain, it mediates cell-cell and cell-matrix adhesion. |
| Signal transduction | The intracellular tail can activate downstream signaling pathways, affect cell proliferation, survival, migration and invasion ability. |
| Promote tumor metastasis | In a wide variety of cancer (such as melanoma) increased, enhanced invasive cancer cells, blood vessels to promote its penetration (leakage) and the formation of metastases. |
| Angiogenesis regulation | Expressed in endothelial cells, it participates in the formation process of new blood vessels and provides nutritional support for tumor growth. |
| Stem cell maintenance | The expression on the surface of some stem cells (such as mesenchymal stem cells) may be involved in the migration and homing process of stem cells. |
The adhesion function of MCAM does not rely on calcium ions, which contrasts with calcium-dependent classical cadherins and highlights its particular advantages in rapid and dynamic cell migration, such as tumor cell invasion.
Applications of MCAM and MCAM Antibody in Literature
1. Yang, et al. "Mcam inhibits macrophage-mediated development of mammary gland through non-canonical Wnt signaling." Nature Communications 15.1 (2024): 36. https://doi.org/10.1038/s41467-023-44338-0
This study found through screening that MCAM is a negative regulatory factor of mammary epithelial cells (MEC). Knockout of MCAM can recruit macrophages through the Il4-Stat6 axis, promote their secretion of Wnt5a, and thereby activate the non-classical Wnt pathway receptor Ryk, thereby enhancing the proliferation and differentiation ability of MEC, revealing a new mechanism by which MCAM regulates breast development through the Wnt5a/Ryk axis.
2. Joshkon, Ahmad, et al. "Role of CD146 (MCAM) in physiological and pathological angiogenesis—contribution of new antibodies for therapy." Biomedicines 8.12 (2020): 633. https://doi.org/10.3390/biomedicines8120633
The article indicates that MCAM is an immunoglobulin superfamily adhesion molecule, and its soluble form plays a key role in physiological and pathological angiogenesis. The levels are significantly elevated in patients with various diseases. Monoclonal antibodies targeting MCAM have become a potential therapeutic strategy to block its pathogenic effects.
3. Luo, Wen, et al. "Combinatorial immunotherapy of anti-MCAM CAR-modified expanded natural killer cells and NKTR-255 against neuroblastoma." Molecular Therapy Oncology 32.4 (2024). https://doi.org/10.1016/j.omton.2024.200894
The article indicates that MCAM is an immunoglobulin superfamily adhesion molecule, and its soluble form plays a key role in physiological and pathological angiogenesis. The levels are significantly elevated in patients with various diseases. Monoclonal antibodies targeting MCAM have become a potential therapeutic strategy to block its pathogenic effects.
4. Wang, Weijun, et al. "Intestinal epithelium-specific Fut2 deficiency promotes colorectal cancer through down-regulating fucosylation of MCAM." Journal of translational medicine 21.1 (2023): 82. https://doi.org/10.1186/s12967-023-03906-0
This study reveals that the Fut2 gene defect promotes the occurrence and development of colorectal cancer (CRC) by down-regulating the fucosylation modification of the MCAM protein. In both in vivo and in vitro experiments, the absence of Fut2 led to a decrease in the fucosylation level of MCAM, thereby enhancing the proliferation, invasion and metastasis capabilities of cancer cells. The results indicate that targeting MCAM glycosylation may be a potential therapeutic strategy for FUT2-deficient CRC.
5. Du, Q Zhang, S Wang, et al. "MCAM is associated with metastasis and poor prognosis in osteosarcoma by modulating tumor cell migration." Journal of Clinical Laboratory Analysis 36.2 (2022): e24214. https://doi.org/10.1002/jcla.24214
This study, through bioinformatics analysis, found that high expression of MCAM was significantly associated with poor prognosis and metastasis risk in patients with osteosarcoma. Functional experiments have confirmed that MCAM can regulate tumor cell migration. The results indicate that MCAM is a potential prognostic biomarker for osteosarcoma, and its expression level is helpful for assessing the risk of metastasis.
Creative Biolabs: MCAM Antibodies for Research
Creative Biolabs specializes in the production of high-quality MCAM antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MCAM Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MCAM antibodies, custom preparations, or technical support, contact us at email.
Reference
- Du, Q Zhang, S Wang, et al. "MCAM is associated with metastasis and poor prognosis in osteosarcoma by modulating tumor cell migration." Journal of Clinical Laboratory Analysis 36.2 (2022): e24214. https://doi.org/10.1002/jcla.24214
Anti-MCAM antibodies
 Loading...
Loading...
Hot products 
-
Rabbit Anti-ALOX5AP Recombinant Antibody (CBXF-1219) (CBMAB-F0750-CQ)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Mouse Anti-ACKR3 Recombinant Antibody (V2-261265) (CBMAB-C1023-LY)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Mouse Anti-CIITA Recombinant Antibody (CBLC160-LY) (CBMAB-C10987-LY)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Mouse Anti-CD164 Recombinant Antibody (CBFYC-0077) (CBMAB-C0086-FY)

-
Mouse Anti-CCND2 Recombinant Antibody (DCS-3) (CBMAB-G1318-LY)

-
Rat Anti-FABP3 Recombinant Antibody (CBXF-2299) (CBMAB-F1612-CQ)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Mouse Anti-BHMT Recombinant Antibody (CBYY-0547) (CBMAB-0550-YY)

-
Mouse Anti-ADIPOR1 Recombinant Antibody (V2-179982) (CBMAB-A1368-YC)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-AHCYL1 Recombinant Antibody (V2-180270) (CBMAB-A1703-YC)

-
Mouse Anti-ARIH1 Recombinant Antibody (C-7) (CBMAB-A3563-YC)

-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

-
Mouse Anti-CORO1A Recombinant Antibody (4G10) (V2LY-1206-LY806)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








