MT1 Antibodies
Background
MT1 belongs to the metallothionein family and is a type of low-molecular-weight protein rich in cysteine that is widely present in eukaryotes. This protein can specifically bind to heavy metal ions such as zinc and copper, and mainly participates in the homeostasis maintenance and detoxification process of metal ions in the body. In liver and kidney tissues, MT1 ensures the normal physiological functions of cells by regulating the storage and release of essential trace elements and neutralizing the toxicity of excessive heavy metals. This protein family was first isolated and identified from horse kidneys by Margoshes and Vallee in 1960. Its unique thiol cluster structure can serve as a classic model for studying the mechanism of metal chaperone and oxidative stress response, providing an important molecular research basis for understanding cellular defense mechanisms, environmental toxicology and metal biology.
Structure of MT1
MT1 (metallothionein 1) is a low-molecular-weight protein with a molecular weight of approximately 6-7 kDa. Its precise molecular weight varies slightly due to species specificity and isomer differences.
| Species | Human | Mouse | Rat | Zebrafish | Bovine |
| Molecular Weight (kDa) | 6.1 | 6.0 | 6.1 | 6.3 | 6.0 |
| Primary Structural Differences | Contains 20 cysteines | Conservative cysteine arrangement | Highly homologous to rats | Retain 12 cysteines | Typical structure of mammals |
This protein is composed of approximately 61 amino acids, including 20 highly conserved cysteine residues, which complex divalent metal ions through their thiol groups. The protein skeleton folds into two independent metal-binding domains (α domain and β domain), forming a unique spherical conformation. The β domain at the N-terminal mainly binds to zinc ions to maintain structural stability, while the α domain at the C-terminal has a higher affinity for copper ions. This coordination center composed of cysteine clusters not only determines its metal binding ability but also makes it exhibit a characteristic absorption spectrum in the ultraviolet region.
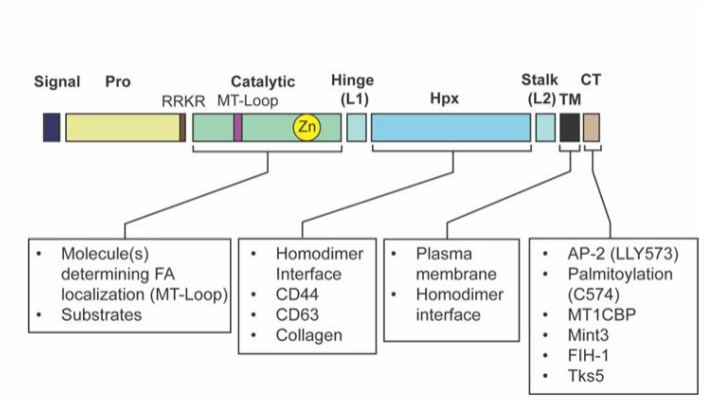 Fig. 1 The domain structure of MT1-MMP is indicated.1
Fig. 1 The domain structure of MT1-MMP is indicated.1
Key structural properties of MT1:
- Unique dual-domain spherical structure
- Metal-bound clusters rich in cysteine
- Binding of divalent metal ions by sulfhydryl coordination bonds
Functions of MT1
The main function of the MT1 gene-encoded protein is to maintain the homeostasis of metal ions within cells and detoxify. In addition, it is also involved in a variety of physiological processes, including oxidative stress responses and cellular protection.
| Function | Description |
| Detoxification of heavy metals | By chelating excessive toxic metals such as cadmium and mercury through cysteine residues, cytotoxicity is reduced. |
| Necessary metal adjustment | Participate in the storage and release of essential trace elements such as zinc and copper, and maintain metabolic balance. |
| Oxidative stress protection | Eliminate free radicals, alleviate the damage of reactive oxygen species (ROS) to cells, and exert antioxidant effects. |
| Regulation of inflammatory response | Expression in the process of immunity and inflammation, cell response to environmental stress. |
| Development and differentiation support | Provides the necessary supply of metal ions for embryonic development and differentiation of certain tissues. |
The MT1 protein has a much higher affinity for heavy metals than essential metals, enabling it to prioritize detoxification when metals are overloaded. This characteristic makes it an important indicator in environmental toxicology and cellular defense research.
Applications of MT1 and MT1 Antibody in Literature
1. Tanaka, Noritaka, and Takeharu Sakamoto. "MT1-MMP as a key regulator of metastasis." Cells 12.17 (2023): 2187. https://doi.org/10.3390/cells12172187
The article indicates that MT1-MMP is a membrane matrix metalloproteinase that promotes cancer invasion and metastasis by degrading the extracellular matrix and activating MMP-2. It can also enhance sugar metabolism through non-protease pathways. Recent research on its specific inhibitors has provided a new direction for cancer treatment.
2. Gu, Chao, et al. "Microglial MT1 activation inhibits LPS‐induced neuroinflammation via regulation of metabolic reprogramming." Aging cell 20.6 (2021): e13375. https://doi.org/10.1111/acel.13375
The article indicates that MT1-MMP promotes cancer invasion and metastasis by degrading the extracellular matrix and activating MMP-2. It can also non-proteinally enhance sugar metabolism. The research on its specific inhibitors provides a new direction for cancer treatment.
3. Liu, Jinlong, et al. "Potential target within the tumor microenvironment-MT1-MMP." Frontiers in Immunology 16 (2025): 1517519. https://doi.org/10.3389/fimmu.2025.1517519
The article indicates that the membrane protease MT1-MMP drives tumor invasion and metastasis by degrading the extracellular matrix. Its overexpression is associated with the poor prognosis of various cancers and is an important therapeutic target. This article reviews the structure, function of MT1-MMP and the current status and prospects of its targeted therapy.
4. Knapinska, Anna M., et al. "Screening MT1-MMP Activity and Inhibition in Three-Dimensional Tumor Spheroids." Biomedicines 11.2 (2023): 562. https://doi.org/10.3390/biomedicines11020562
In this study, a tumor sphere model based on collagen hydrogel was developed, and its enzymatic activity was quantified using MT1-MMP-specific fluorescent substrates. This model confirmed that glioma invasion depends on MT1-MMP and can screen its inhibitors in high throughput, providing an efficient platform for the research and development of anti-cancer drugs.
5. Suárez, Henar, et al. "Regulation of MT1-MMP activity through its association with ERMs." Cells 9.2 (2020): 348. https://doi.org/10.3390/cells9020348
Research has found that MT1-MMP binds to ERM cytoskeletal junction proteins through a positive charge cluster in its intracellular segment. This interaction dominates the self-processing of MT1-MMP on the cell membrane and its collagen degradation activity, thereby regulating the key function of this enzyme in tumor cell invasion.
Creative Biolabs: MT1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality MT1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MT1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MT1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Kelly, Hannah, Masaki Inada, and Yoshifumi Itoh. "The diverse pathways for cell surface MT1-MMP localization in migratory cells." Cells 14.3 (2025): 209. https://doi.org/10.3390/cells14030209
Anti-MT1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AOC3 Recombinant Antibody (CBYY-0014) (CBMAB-0014-YY)

-
Mouse Anti-ATP1B3 Recombinant Antibody (1E9) (CBMAB-A4021-YC)

-
Mouse Anti-AK4 Recombinant Antibody (V2-180419) (CBMAB-A1891-YC)

-
Mouse Anti-ACTN4 Recombinant Antibody (V2-6075) (CBMAB-0020CQ)

-
Mouse Anti-ARSA Recombinant Antibody (CBYC-A799) (CBMAB-A3679-YC)

-
Mouse Anti-ALDOA Recombinant Antibody (A2) (CBMAB-A2316-YC)

-
Rabbit Anti-CCN1 Recombinant Antibody (CBWJC-3580) (CBMAB-C4816WJ)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Armenian hamster Anti-CD40 Recombinant Antibody (HM40-3) (CBMAB-C10365-LY)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Mouse Anti-CARD11 Recombinant Antibody (CBFYC-0811) (CBMAB-C0866-FY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-CCL18 Recombinant Antibody (64507) (CBMAB-C7910-LY)

-
Mouse Anti-ABIN2 Recombinant Antibody (V2-179106) (CBMAB-A0349-YC)

-
Mouse Anti-ADAM12 Recombinant Antibody (V2-179752) (CBMAB-A1114-YC)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Mouse Anti-CTCF Recombinant Antibody (CBFYC-2371) (CBMAB-C2443-FY)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








