VEGFA Antibodies
Background
VEGFA is a secretory glycoprotein dimer that is widely expressed in vascular endothelial cells and various tissue cells. The protein encoded by this gene, as a key angiogenic factor, specifically binds to VEGFR receptors on the cell membrane surface, activates downstream signaling pathways, and thereby promotes the proliferation, migration of vascular endothelial cells and changes in vascular permeability. During embryonic development and trauma repair, VEGFA maintains the balance of tissue oxygen supply by coordinating angiogenesis. This gene was first identified by the Napoleone Ferrara team in 1989. The analysis of its three-dimensional structure revealed the specific mechanism of the interaction between growth factors and receptors. The related research won the Lasker Award for Clinical Medical Research in 2010. VEGFA, as a core molecule in vascular biology research, its regulatory mechanism has become an important theoretical basis for tumor treatment and drug development for ischemic diseases.
Structure of VEGFA
VEGFA is an important secretory signaling protein. Its molecular weight varies significantly due to transcript differences, and the common subtype range is approximately 22-45 kDa. Different subtypes have significant differences in amino acid composition and function.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | VEGFA165 (45 kDa) | VEGFA164 (44 kDa) | VEGFA164 (44 kDa) |
| Primary Structural Differences | The heparin-binding domain is retained | Highly homologous to human VEGFA165 | Enhanced regulation of vascular permeability |
This protein is formed by two identical subunits linked by disulfide bonds to create a homodimer. Its core functional area includes the receptor-binding domain, which can specifically recognize VEGFR1/2. Key cysteine residues (such as Cys51 and Cys60) are crucial for the formation and stability of the dimer structure. Residues such as arginine (Arg82) on the receptor binding interface directly participate in the charge interaction with the receptor, while specific amino acid variations located in the signal peptide region will affect the secretion efficiency of the protein.
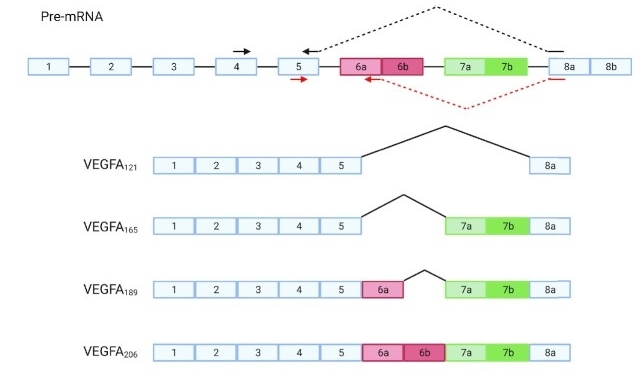 Fig. 1 Schematic representation of the VEGFA gene and four of its known isoforms.1
Fig. 1 Schematic representation of the VEGFA gene and four of its known isoforms.1
Key structural properties of VEGFA:
- Characteristic cystine knot structural core
- Alkaline amino acid rich region at the receptor binding interface
- Carboxyl-terminal heparin binding domain (major subtype)
Functions of VEGFA
The core function of VEGFA is to promote angiogenesis and enhance vascular permeability, while also participating in the regulation of various pathophysiological processes.
| Function | Description |
| Angiogenesis | Induce the proliferation, migration and formation of new blood vessels of endothelial cells, providing a basis for tissue growth and repair. |
| Regulation of vascular permeability | Enhance the permeability of microvessels, promote the extravasation of plasma proteins, and provide a temporary matrix for angiogenesis. |
| Cell survival and maintenance | By activating anti-apoptotic signaling pathways, it supports the survival of vascular endothelial cells under stressful conditions. |
| Inflammatory regulation | Coordinate the recruitment of inflammatory cells in the trauma or ischemic area and form a feedback regulatory network with inflammatory factors. |
| Tumor vascularization | Overexpression in the tumor microenvironment induces abnormal vascular proliferation, promoting tumor growth and metastasis. |
The signal activation of VEGFA shows dose-dependent characteristics, which is completely different from the cooperative oxygen binding of hemoglobin, demonstrating its precise regulatory ability on angiogenesis under physiological and pathological conditions.
Applications of VEGFA and VEGFA Antibody in Literature
1. White, Amanda Louise, and Gregory Jaye Bix. "VEGFA isoforms as pro-angiogenic therapeutics for cerebrovascular diseases." Biomolecules 13.4 (2023): 702. https://doi.org/10.3390/biom13040702
The article indicates that VEGFA can promote cerebral vascular formation in animal experiments, but the clinical trials have shown poor results due to the administration method and its increase in vascular permeability. Due to their different mechanisms of action, their various subtypes are expected to become potential therapies for treating cerebrovascular diseases.
2. Wang, Lu, et al. "VEGFA/NRP-1/GAPVD1 axis promotes progression and cancer stemness of triple-negative breast cancer by enhancing tumor cell-macrophage crosstalk." International Journal of Biological Sciences 20.2 (2024): 446. https://doi.org/10.7150/ijbs.86085
Research has found that in triple-negative breast cancer, M2-type macrophages form a feedback loop with cancer cells by secreting VEGFA. VEGFA enhances the stem cell characteristics of cancer cells through the NRP-1/GAPVD1 axis and creates an immunosuppressive microenvironment. This provides a new idea for the combination of anti-VEGFA and immunotherapy.
3. Zhang, Lei, et al. "CircRNA-miRNA-VEGFA: an important pathway to regulate cancer pathogenesis." Frontiers in Pharmacology 14 (2023): 1049742. https://doi.org/10.3389/fphar.2023.1049742
Research has found that circular Rnas (circrnas) regulate the expression of vascular endothelial growth factor A (VEGFA) during the course of cancer by adsorbing mirnas as molecular "sponges". VEGFA is a key factor driving tumor angiogenesis. Therefore, the circRNA-VEGFA axis provides a new perspective for understanding cancer pathogenesis.
4. Dahan, Sara, et al. "VEGFA's distal enhancer regulates its alternative splicing in CML." Nar Cancer 3.3 (2021): zcab029. https://doi.org/10.1093/narcan/zcab029
Research has found that in chronic myeloid leukemia, the specific enhancer downstream of the VEGFA gene is activated through demethylation. This enhancer recruits the CCNT2 protein to accelerate the rate of transcriptional extension, thereby regulating the alternative splicing of VEGFA itself, promoting the inclusion of specific exons related to cancer, and ultimately influencing the pathological process of leukemia.
5. Comunanza, Valentina, et al. "Dual VEGFA/BRAF targeting boosts PD‐1 blockade in melanoma through GM‐CSF‐mediated infiltration of M1 macrophages." Molecular Oncology 17.8 (2023): 1474-1491. https://doi.org/10.1002/1878-0261.13450
Research has found that in the treatment of melanoma with BRAFV600E, the combined inhibition of VEGFA and BRAF can reshape the tumor microenvironment, promote the infiltration of M1 macrophages and CD8+ T cells, and enhance the tumor's sensitivity to immune checkpoint blockade. This combined strategy provides a new basis for targeted VEGFA and immunotherapy.
Creative Biolabs: VEGFA Antibodies for Research
Creative Biolabs specializes in the production of high-quality VEGFA antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom VEGFA Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our VEGFA antibodies, custom preparations, or technical support, contact us at email.
Reference
- Dahan, Sara, et al. "VEGFA's distal enhancer regulates its alternative splicing in CML." Nar Cancer 3.3 (2021): zcab029. https://doi.org/10.1093/narcan/zcab029
Anti-VEGFA antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

-
Mouse Anti-APP Recombinant Antibody (DE2B4) (CBMAB-1122-CN)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Mouse Anti-CARTPT Recombinant Antibody (113612) (CBMAB-C2450-LY)

-
Mouse Anti-FLT1 Recombinant Antibody (11) (CBMAB-V0154-LY)

-
Rat Anti-C5AR1 Recombinant Antibody (8D6) (CBMAB-C9139-LY)

-
Mouse Anti-ACVR1C Recombinant Antibody (V2-179685) (CBMAB-A1041-YC)

-
Mouse Anti-CD83 Recombinant Antibody (HB15) (CBMAB-C1765-CQ)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Mouse Anti-ENO1 Recombinant Antibody (CBYC-A950) (CBMAB-A4388-YC)

-
Mouse Anti-ARSA Recombinant Antibody (CBYC-A799) (CBMAB-A3679-YC)

-
Mouse Anti-EIF4G1 Recombinant Antibody (2A9) (CBMAB-A2544-LY)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Rabbit Anti-AP2M1 (Phosphorylated T156) Recombinant Antibody (D4F3) (PTM-CBMAB-0610LY)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-BSN Recombinant Antibody (219E1) (CBMAB-1228-CN)

-
Mouse Anti-APOE Recombinant Antibody (A1) (CBMAB-0078CQ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








