XDH Antibodies
Background
The XDH gene encodes xanthine dehydrogenase, a molybdenum-containing protease mainly present in the liver and intestinal mucosa. This enzyme catalyzes the conversion of hypoxanthine to xanthine in the purine metabolic pathway and further converts xanthine into uric acid, while also participating in the generation and regulation of reactive oxygen species. Due to its core role in uric acid production, abnormal expression or activity changes of XDH are closely related to various diseases such as gout, kidney stones and ischemia-reperfusion injury. This gene was first identified through biochemical research in the 1950s, and the three-dimensional structure of its encoded protein was resolved by X-ray crystallography in the early 21st century, which deepened people's understanding of the mechanism of molybdenum cofactor-dependent oxidoreductase. The research on the molecular structure and function of XDH continuously provides a key theoretical basis for the treatment of metabolic diseases and antioxidant strategies.
Structure of XDH
Xanthine dehydrogenase (XDH) encoded by the XDH gene is a homodimer protein with a molecular weight of approximately 150 kDa. There are certain differences in its molecular weight among different species, which mainly result from the subtle changes in the structure of the enzyme protein.
| Species | Human | Mouse | Bovine |
| Molecular Weight (kDa) | ~150 | ~145 | ~155 |
| Primary Structural Differences | Conservative catalytic core, containing methotrexate cofactor | The structures are highly similar and the active sites are highly conserved | There is a small amount of non-critical amino acid substitution |
The primary structure of the XDH protein is composed of approximately 1,333 amino acid residues, which fold into a complex multi-domain structure. Its advanced structure consists of three main functional domains: two iron-sulfur cluster (2Fe-2S) domains, one flavin adenine dinucleotide (FAD) binding domain, and a key molybdenum methoxate cofactor (Mo-co) catalytic center. These domains together form a precise electron transport chain: when catalyzing the oxidation of purine substrates (such as hypoxanthine), electrons are successively transferred from the molybdenum center to the iron-sulfur cluster, and finally reach FAD, where electrons are transferred to coenzyme NAD⁺ or molecular oxygen, thereby completing the REDOX reaction. The conformational transformation of this enzyme between the oxidized form (dehydrogenase, XDH) and the reduced form (oxidase, XO) is the key to regulating its physiological and pathological functions.
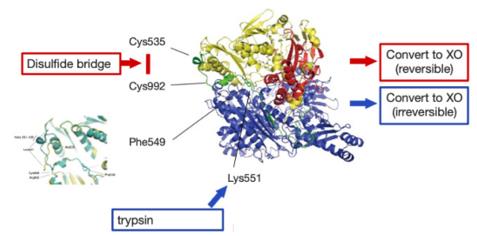 Fig. 1 Illustration of the mechanism of conversion from XDH to XO.1
Fig. 1 Illustration of the mechanism of conversion from XDH to XO.1
Key structural properties of XDH:
- Multi-domain homodimer configuration
- Sulfur clusters containing molybdenum poison, iron and flavin adenine dinucleotide (FAD) three REDOX center
- Fe-sulfur cluster and FAD domain form electron transport chain
- Glutamate residues at key catalytic sites coordinate molybdenum cofactors for substrate oxidation
Functions of XDH
The core function of xanthine dehydrogenase (XDH, encoded by the XDH gene) is to catalyze the final step of purine metabolism. However, it is also widely involved in various physiological and pathological processes such as REDOX balance, reactive oxygen species generation and inflammatory responses.
| Function | Description |
| Purine metabolism | As a key enzyme in purine catabolism, it successively catalyzes the oxidation of hypoxanthine to xanthine and xanthine to uric acid. |
| Reactive oxygen species generation | Under specific conditions (such as ischemia-reperfusion), its oxidase form (XO) can directly use molecular oxygen as the electron acceptor to produce reactive oxygen species (such as superoxide anion, hydrogen peroxide). |
| REDOX signal regulation | Products through its enzyme activity (e.g., uric acid, reactive oxygen species) involved in the REDOX signaling inside the cell, a variety of physiological processes. |
| Inflammation and tissue damage | The reactive oxygen species it generates are important factors mediating ischemia-reperfusion injury, chronic inflammation and endothelial dysfunction. |
| Drug metabolism | To participate in some nitrogen heterocyclic drugs (such as antitumor drug 6-mercaptopurine) oxidative metabolism. |
The catalytic mechanism of XDH involves a precise electron transport chain: electrons are transferred from the substrate through the molybdenum cofactor, successively to the two iron-sulfur clusters, and finally reach FAD. Its unique dual-functional characteristics - which can be manifested as NAD⁺-dependent dehydrogenase (XDH) and also transformed into oxygen-dependent oxidase (XO) - form the structural basis for its participation in various physiological and pathological processes.
Applications of XDH and XDH Antibody in Literature
- Nishino, Takeshi. "XDH and XO research and drug discovery—Personal history." Molecules 28.11 (2023): 4440. https://doi.org/10.3390/molecules28114440
This article summarizes the molecular mechanism of the conversion of xanthine dehydrogenase (XDH) to oxidase (XO) and its physiological and pathological significance. Relevant inhibitors have been successfully developed for the treatment of gout. The author's research over the past five decades has covered enzymatic properties, transformation mechanisms and drug development.
- Liang, Ningning, et al. "Fatty acid oxidation-induced HIF-1α activation facilitates hepatic urate synthesis through upregulating NT5C2 and XDH." Life metabolism 3.5 (2024): loae018. https://doi.org/10.1093/lifemeta/loae018
Research has found that fatty acid oxidation directly links lipid metabolism disorders with hyperuricemia by activating HIF-1α, upregulating NT5C2 and XDH, promoting the liver's uptake of hypoxanthine and synthesis of uric acid.
- Snoozy, Jennifer, et al. "XDH-1 inactivation causes xanthine stone formation in Caenorhabditis elegans which is inhibited by SULP-4-mediated anion exchange in the excretory cell." PLoS Biology 23.9 (2025): e3003410. https://doi.org/10.1371/journal.pbio.3003410
In this study, a nematode model was used to explore XDH deficiency. It was found that in addition to the loss of XDH function, the sulfate transporter SULP-4 is also a key factor affecting the formation of xanthine stones by regulating osmotic pressure imbalance.
- Wang, Yiting, et al. "C1QBP regulates apoptosis of renal cell carcinoma via modulating xanthine dehydrogenase (XDH) mediated ROS generation." International Journal of Medical Sciences 19.5 (2022): 842. https://doi.org/10.7150/ijms.71703
Research has found that C1QBP induces apoptosis of renal cancer cells by up-regulating the expression of XDH, promoting the catabolism of hypoxanthine and generating ROS, thereby revealing its key role in the metabolism and oxidative stress of renal cancer.
- Starr, Lindsey A., et al. "Attenuation of dopaminergic neurodegeneration in a C. elegans Parkinson's model through regulation of xanthine dehydrogenase (XDH-1) expression by the RNA Editase, ADR-2." Journal of Developmental Biology 11.2 (2023): 20. https://doi.org/10.3390/jdb11020020
Research has found that in the nematode model, the expression of WHT-2 is regulated through RNA editing mediated by ADAR2, which affects the excretion of uric acid and then negatively inhibits XDH-1/XO to reduce the production of reactive oxygen species, thereby providing protection for dopaminergic neurons.
Creative Biolabs: XDH Antibodies for Research
Creative Biolabs specializes in the production of high-quality XDH antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom XDH Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our XDH antibodies, custom preparations, or technical support, contact us at email.
Reference
- Nishino, Takeshi. "XDH and XO research and drug discovery—Personal history." Molecules 28.11 (2023): 4440. https://doi.org/10.3390/molecules28114440
Anti-XDH antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Mouse Anti-dsRNA Recombinant Antibody (2) (CBMAB-D1807-YC)

-
Mouse Anti-ALB Recombinant Antibody (V2-363290) (CBMAB-S0173-CQ)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Mouse Anti-ACKR3 Recombinant Antibody (V2-261265) (CBMAB-C1023-LY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-CRYAB Recombinant Antibody (A4345) (CBMAB-A4345-YC)

-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

-
Mouse Anti-CD24 Recombinant Antibody (2Q1282) (CBMAB-C1624-CN)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-CD46 Recombinant Antibody (CBFYC-0076) (CBMAB-C0085-FY)

-
Mouse Anti-dsDNA Recombinant Antibody (22) (CBMAB-AP1954LY)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Mouse Anti-CCDC6 Recombinant Antibody (CBXC-0106) (CBMAB-C5397-CQ)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Rabbit Anti-BAD (Phospho-Ser136) Recombinant Antibody (CAP219) (CBMAB-AP536LY)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








