CANX Antibodies
Background
The CANX gene encodes an endoplasmic reticulum transmembrane chaperone protein called calnexin, which is mainly present in the endoplasmic reticulum of eukaryotic cells. It maintains cellular protein homeostasis by recognizing and binding to newly synthesized glycoproteins, assisting in their correct folding and assembly, and participating in quality control mechanisms to ensure that misfolded proteins are degraded. CANX plays a key role in a variety of cellular processes, especially in immune responses and cell adhesion, as many immune receptors and adhesion molecules rely on it to assist in maturation. This gene was identified in the early 1990s. Its functional research has revealed an important mechanism of the protein processing and quality control pathway in the endoplasmic reticulum, providing an important foundation for understanding protein folding abnormalities related to certain genetic diseases and cancers.
Structure of CANX
Calnexin, encoded by the CANX gene, is an endoplasmic reticulum transmembrane protein with a molecular weight of approximately 90 kDa. Its molecular weight is highly conserved among different species, which is closely related to its core function in the quality control of protein folding.
| Species | Human | Mouse | Bovine | Rat |
| Molecular Weight (kDa) | 90 | 90 | 89.5 | 90 |
| Primary Structural Differences | Contains an N-terminal lectin domain, a single transmembrane region, and a C-terminal cytoplasmic tail | Highly homologous to humans and structurally highly similar | Highly conservative agglutinin structure domain | Consistent with the basic human function structure domain |
Calcium-junction proteins contain 592 amino acids, and their primary structure forms a typical type I transmembrane protein. The core of this protein is its N-terminal lectin-like domain, which specifically recognizes and binds to the monosaccharide glucose residue on the newly synthesized glycoprotein. This is the structural basis for it to perform its molecular chaperone function. A single transmembrane helix anchors it to the endoplasmic reticulum membrane, while the cytoplasmic tail at the C-terminal contains endoplasmic reticulum retention signals.
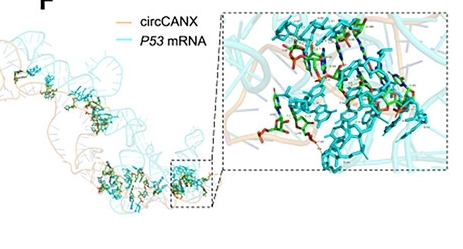 Fig. 1 Simulated interaction between circCANX and P53 transcript.1
Fig. 1 Simulated interaction between circCANX and P53 transcript.1
Key structural properties of CANX:
- Unique topological structure
- Conservative lectin domain
- Repeat sequences rich in proline
- Transmembrane anchoring and cytoplasmic tail
Functions of CANX
The Calnexin encoded by the CANX gene mainly functions as a molecular chaperone in the endoplasmic reticulum, assisting in the correct folding and assembly of glycoproteins. In addition, it is also deeply involved in key cellular quality control processes such as endoplasmic reticulum associated degradation (ERAD).
| Function | Description |
| Promote glycoprotein folding | Its N-terminal lectin domain specifically recognizes Glc₁Man₉GlcNAc₂glycan on the newly synthesized glycoprotein, binds to it and provides a folding environment. |
| Quality control monitoring | Synergistic with the co-chaperone ERp57, which repeatedly binds to incompletely folded glycoproteins, ensures that only correctly folded proteins are transported out of the ER. |
| Target misfolded proteins | Identify and retain proteins that fail to fold, guide them to the endoplasmic reticulum associated degradation (ERAD) pathway for clearance, and prevent the accumulation of incorrect proteins. |
| Calcium ion homeostasis is maintained | As a calcium-binding protein within the endoplasmic reticulum lumen, its function depends on the concentration of calcium ions and indirectly participates in the regulation of intracellular calcium homeostasis. |
| Regulation of apoptosis | By affecting the endoplasmic reticulum stress response, it plays a regulatory role in the signaling pathway of cell apoptosis. |
The functions of Calreticulin and the homologous protein calreticulin complement each other. However, as a transmembrane protein, calreticulin particularly focuses on the folding monitoring of membrane proteins and some secreted proteins. Its mechanism of action reflects the high specificity and accuracy of the endoplasmic reticulum quality control system.
Applications of CANX and CANX Antibody in Literature
1. Ye, Yuanzheng, et al. "PON2 ameliorates Ang II‐induced cardiomyocyte injury by targeting the CANX/NOX4 signaling pathway." Immunity, Inflammation and Disease 11.2 (2023): e765. https://doi.org/10.1002/iid3.765
This study explored the role of PON2 in angiotensin II-induced myocardial cell injury. It was found that PON2 regulates the NOX4 signaling pathway by targeting CANX, thereby inhibiting oxidative stress, inflammation and cell hypertrophy, and exerting a protective effect on cardiomyocytes.
2. Liu, Xuchen, et al. "Calnexin promotes glioblastoma progression by inducing protective mitophagy through the MEK/ERK/BNIP3 pathway." Theranostics 15.6 (2025): 2624. https://doi.org/10.7150/thno.105591
This study confirmed that the endoplasmic reticulum protein CANX promotes BNIP3-mediated mitochondrial autophagy in glioblastoma by activating the MEK/ERK signaling pathway, thereby enhancing the resistance of tumor cells to temozolomide. Targeting CANX can effectively reverse chemotherapy resistance, suggesting its potential as a new therapeutic target.
3. Chen, Ting‐Ting, et al. "ADAR1‐HNRNPL‐Mediated CircCANX Decline Promotes Autophagy in Chronic Obstructive Pulmonary Disease." Advanced Science 12.18 (2025): 2414211. https://doi.org/10.1002/advs.202414211
This study found that in chronic obstructive pulmonary disease, the expression of circular RNA circCANX is down-regulated. circCANX mediates P53 mRNA degradation by recruiting UPF1, thereby inhibiting autophagy and the formation of stress granules, and exacerbating airway inflammation. circCANX has the potential to be a therapeutic target and biomarker for COPD.
4. Shapiro, Ilja E., et al. "Deleterious knock-outs in the HLA class I antigen processing and presentation machinery induce distinct changes in the immunopeptidome." Molecular & Cellular Proteomics (2025): 100951. https://doi.org/10.1016/j.mcpro.2025.100951
In this study, a HAP1 cell line gene knockout model was constructed to systematically analyze the impact of the absence of key components of the antigen processing and presentation mechanism (APPM) on the immunopeptide group. Research has found that knocking out genes such as CANX can lead to significant alterations in HLA allele-specific presenting peptides, providing a new perspective for understanding antigen presentation deficits in disease conditions.
5. Vélez-López, Omar, et al. "Analysis of Sigma-1 Receptor Antagonist BD1047 Effect on Upregulating Proteins in HIV-1-Infected Macrophages Exposed to Cocaine Using Quantitative Proteomics." Biomedicines 12.9 (2024): 1934. https://doi.org/10.3390/biomedicines12091934
This study explored the role of Sig1R antagonist BD1047 in HIV-infected macrophages exposed to cocaine. The results indicated that pretreatment with BD1047 could up-regulate endoplasmic reticulum transportation-related proteins such as CANX, alleviate mitochondrial damage and CatB-mediated neurotoxicity, and exert protective effects.
Creative Biolabs: CANX Antibodies for Research
Creative Biolabs specializes in the production of high-quality CANX antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom CANX Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our CANX antibodies, custom preparations, or technical support, contact us at email.
Reference
- Chen, Ting‐Ting, et al. "ADAR1‐HNRNPL‐Mediated CircCANX Decline Promotes Autophagy in Chronic Obstructive Pulmonary Disease." Advanced Science 12.18 (2025): 2414211. https://doi.org/10.1002/advs.202414211
Anti-CANX antibodies
 Loading...
Loading...
Hot products 
-
Armenian hamster Anti-CD40 Recombinant Antibody (HM40-3) (CBMAB-C10365-LY)

-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

-
Rabbit Anti-ALOX5AP Recombinant Antibody (CBXF-1219) (CBMAB-F0750-CQ)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

-
Mouse Anti-CAT Recombinant Antibody (724810) (CBMAB-C8431-LY)

-
Mouse Anti-ACVR1C Recombinant Antibody (V2-179685) (CBMAB-A1041-YC)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Mouse Anti-C5AR1 Recombinant Antibody (R63) (CBMAB-C9553-LY)

-
Mouse Anti-BIRC3 Recombinant Antibody (315304) (CBMAB-1214-CN)

-
Mouse Anti-AAV8 Recombinant Antibody (V2-634028) (CBMAB-AP022LY)

-
Mouse Anti-CD33 Recombinant Antibody (P67.6) (CBMAB-C10189-LY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-CCDC6 Recombinant Antibody (CBXC-0106) (CBMAB-C5397-CQ)

-
Mouse Anti-BRD3 Recombinant Antibody (CBYY-0801) (CBMAB-0804-YY)

-
Mouse Anti-BSN Recombinant Antibody (219E1) (CBMAB-1228-CN)

-
Mouse Anti-BCL2L1 Recombinant Antibody (H5) (CBMAB-1025CQ)

-
Rabbit Anti-ABL1 (Phosphorylated Y185) Recombinant Antibody (V2-443434) (PTM-CBMAB-0001YC)

-
Rat Anti-CCR2 Recombinant Antibody (475301) (CBMAB-C1338-LY)

-
Rabbit Anti-CCL5 Recombinant Antibody (R0437) (CBMAB-R0437-CN)

-
Mouse Anti-ADGRL2 Recombinant Antibody (V2-58519) (CBMAB-L0166-YJ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot