CIITA Antibodies
Background
CIITA, as a key transcriptional regulatory protein, mainly exists in the immune cells of mammals. The protein encoded by this gene plays a core role in adaptive immune responses by activating the expression of MHC Class II molecules, helping the body recognize and eliminate pathogens. Its functional deficiency can lead to severe immune deficiency diseases, such as type II naked lymphocyte syndrome. Since its discovery in 1993, CIITA has been widely studied due to its core regulatory role in the immune system, becoming an important model for understanding the regulation of immune gene expression, epigenetic modifications, and the mechanisms of autoimmune diseases, providing a theoretical basis for the development of related treatment strategies.
Structure of CIITA
CIITA is a transcriptional regulatory protein with a molecular weight of approximately 130 kDa, and its size varies slightly depending on different alternative splicing subtypes such as CiITA-I, III, and IV. This protein contains multiple functional regions such as the N-terminal acidic domain, GTP-binding domain, proline-serine-threonine-rich domain, and C-terminal leucine-zipper domain. These structures together enable it to act as a "master regulatory factor", specifically binding to and activating the transcription of MHC class II genes. Thus, it plays an indispensable core role in adaptive immune responses.
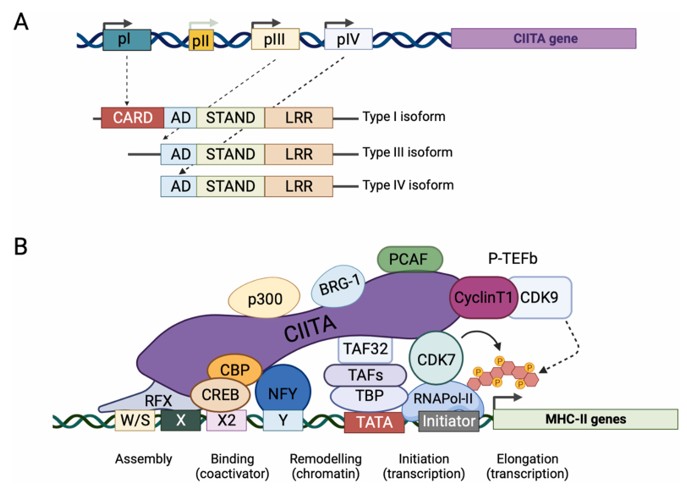 Fig. 1 The MHC class II transactivator CIITA: schematic structure of the gene and function of the protein.1
Fig. 1 The MHC class II transactivator CIITA: schematic structure of the gene and function of the protein.1
Key structural properties of CIITA:
- Modular multi-domain configuration
- The C-terminal LRR-mediated protein interaction interface
- Functional verified location signal
Functions of CIITA
The main function of CIITA is to act as the primary regulatory factor for MHC Class II gene transcription and control adaptive immune responses. It is also involved in regulating key physiological processes such as inflammatory responses and autoimmune tolerance.
| Function | Description |
| MHC II transcriptional activation | CIITA, through its protein domain, directly recruits histone modification enzymes and universal transcription factors to the promoters of MHC class II genes as molecular Bridges, initiating their transcription. |
| Coordination of immune response | By uniformly up-regulating all MHC Class II molecules and related antigen-processing presentation elements, the effective activation of CD4+ T cells is coordinated. |
| Regulation of immune tolerance | Regulation of MHC II expression in specific cells such as thymic stromal cells is involved in the selection of T cells and the establishment of autoimmune tolerance. |
| Integration of inflammatory signals | Its expression is precisely regulated by cytokine signals such as IFN-γ, converting extracellular immune signals into accurate gene transcription responses. |
| Association with immune diseases | The loss of its function leads to severe combined immune deficiency (such as type II naked lymphocyte syndrome), and its abnormal expression is associated with some autoimmune diseases. |
Unlike transcription factors that typically have a single binding site, CIITA assemples multi-protein complexes through its modular domain, which endows its regulatory curve with synergy and high specificity, ensuring that immune genes are activated in a timely and adequate manner only in the desired cells.
Applications of CIITA and CIITA Antibody in Literature
1. Forlani, Greta, et al. "The NLR member CIITA: Master controller of adaptive and intrinsic immunity and unexpected tool in cancer immunotherapy." biomedical journal 46.5 (2023): 100631. https://doi.org/10.1016/j.bj.2023.100631
The article indicates that CIITA is a pioneering member of the NLR family. As a core regulatory factor of MHC-II gene expression, it plays a dual role in innate immunity and adaptive immunity: it is not only a limiting factor for human retroviruses but also can induce cancer cells to express MHC-II, making them antigen-presenting cells for tumor antigens.
2. León Machado, Jorge Alfonso, and Viktor Steimle. "The MHC class II transactivator CIITA: not (quite) the odd-one-out anymore among NLR proteins." International journal of molecular sciences 22.3 (2021): 1074. https://doi.org/10.3390/ijms22031074
The article indicates that CIITA is a key regulatory factor for MHC-II gene expression and functions as a retroviral limiting factor in innate immunity. Recent studies have found that its homologous protein NLRC5 can regulate MHC-I, and both activate the expression of immune-related genes in a non-DNA binding manner.
3. Liu, Huan, et al. "Osteocyte CIITA aggravates osteolytic bone lesions in myeloma." Nature communications 13.1 (2022): 3684. https://doi.org/10.1038/s41467-022-31356-7
The article indicates that in multiple myeloma, enhanced bone resorption mediated by osteoclasts and reduced bone formation mediated by osteoblasts jointly lead to osteolytic lesions. This study found that CIITA expressed by bone cells regulates the expression of RANKL and sclerosing proteins through epigenetics, promoting the osteolytic process of multiple myeloma. Its own expression is upregulated by 2-deoxyd-ribose secreted by multiple myeloma cells via the STAT1/IRF1 pathway.
4. Rane, Grishma, et al. "ZBTB48 is a priming factor regulating B-cell-specific CIITA expression." The EMBO Journal 43.24 (2024): 6236-6263. https://doi.org/10.1038/s44318-024-00306-y
This study reveals that the telomere-binding protein ZBTB48 regulates the CIITA/MHC-II expression program by directly binding to the core activating element of the B-cell specific promoter pIII. Deletion of ZBTB48 blocks IFN-γ -induced CIITA expression and leads to MHC-II silencing in mouse B cells, indicating that ZBTB48 exerts the molecular switch function of B-cell-specific CIITA expression by promoting chromatin opening in the pIII region.
5. Salvato, Ilaria, et al. "Adenoviral delivery of the CIITA transgene induces T‐cell‐mediated killing in glioblastoma organoids." Molecular Oncology 19.3 (2025): 682-697. https://doi.org/10.1002/1878-0261.13750
In this study, CIITA was delivered to glioblastoma (GB) via adenovirus, successfully inducing tumor cells to express MHC-II molecules. When co-cultured with immune cells, regardless of whether CIITA can effectively induce MHC-II or not, it can induce significant T-cell-mediated tumor cell killing. This indicates that CIITA may enhance T-cell immunity through a mechanism partially independent of MHC-II, providing a new strategy for GB immunotherapy.
Creative Biolabs: CIITA Antibodies for Research
Creative Biolabs specializes in the production of high-quality CIITA antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom CIITA Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our CIITA antibodies, custom preparations, or technical support, contact us at email.
Reference
- Forlani, Greta, et al. "The NLR member CIITA: Master controller of adaptive and intrinsic immunity and unexpected tool in cancer immunotherapy." biomedical journal 46.5 (2023): 100631. https://doi.org/10.1016/j.bj.2023.100631
Anti-CIITA antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CTNND1 Recombinant Antibody (CBFYC-2414) (CBMAB-C2487-FY)

-
Mouse Anti-CCN2 Recombinant Antibody (CBFYC-2383) (CBMAB-C2456-FY)

-
Mouse Anti-ENO1 Recombinant Antibody (CBYC-A950) (CBMAB-A4388-YC)

-
Mouse Anti-C1QC Recombinant Antibody (CBFYC-0600) (CBMAB-C0654-FY)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Mouse Anti-CD24 Recombinant Antibody (ALB9) (CBMAB-0176CQ)

-
Mouse Anti-C5AR1 Recombinant Antibody (R63) (CBMAB-C9553-LY)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Mouse Anti-AGK Recombinant Antibody (V2-258056) (CBMAB-M0989-FY)

-
Mouse Anti-CORO1A Recombinant Antibody (4G10) (V2LY-1206-LY806)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

-
Rabbit Anti-BRCA2 Recombinant Antibody (D9S6V) (CBMAB-CP0017-LY)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Rabbit Anti-Acetyl-Histone H3 (Lys36) Recombinant Antibody (V2-623395) (CBMAB-CP0994-LY)

-
Mouse Anti-ARIH1 Recombinant Antibody (C-7) (CBMAB-A3563-YC)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CR3022) (CBMAB-CR014LY)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








