DLST Antibodies
Background
The DLST gene encodes dihydrothiocarcinamide succinyltransferase, which exists as a core component of the mammalian mitochondrial α -ketoglutarate dehydrogenase complex. This protein directly participates in the metabolic regulation of the tricarboxylic acid cycle by catalyzing the transfer of succinyl groups from thioacyl to coenzyme A, maintaining the homeostasis of cellular energy metabolism. The abnormal expression of DLST in tumor cells is closely related to the dysfunction of the mitochondrial respiratory chain, as it directly affects the production efficiency of acetyl-CoA. This gene was first located on human chromosome 14 in 1992. The protein structure encoded by it was analyzed by cryo-electron microscopy in 2007, revealing the unique spatial conformation of the domain of the enzyme's active center. As a key molecule in energy metabolism pathways, DLST has become an important model for studying the association between metabolic reprogramming and diseases, providing a molecular basis for exploring pathological mechanisms such as cancer and neurodegenerative diseases.
Structure of DLST
The DLST gene-encoded dihydrothiolipoamide succinyltransferase is a mitochondrial matrix protein with a molecular weight of approximately 48 kDa. There are moderate molecular weight differences of this protein among different species, mainly due to the evolutionary divergence of its nuclear localization signal sequence and enzyme activity region.
| Species | Human | Mouse | Bovine | Zebrafish | Yeast |
| Molecular Weight (kDa) | 48.3 | 48.1 | 48.5 | 49.2 | 51.8 |
| Primary Structural Differences | Contains the typical E2 core domain | High homology with humans | Catalytic sites highly conservative | There is an N-terminal extension sequence | With a unique subunit assembly interface |
This protein is composed of approximately 450 amino acid residues, and its tertiary structure exhibits the classic E2 core enzyme folding pattern. The core functional domain of the DLST protein contains a thioacyl binding site, which is coupled to the succinyl transfer active center through a flexible lysine residue linking domain. Its active center is composed of a highly conserved histidine - glutamic acid diad, which is responsible for catalyzing the transfer reaction of the thioacyl group. The hydrophobic pocket formed around the active center precisely regulates substrate specificity, ensuring the stability of the tricarboxylic acid cycle metabolic flux.
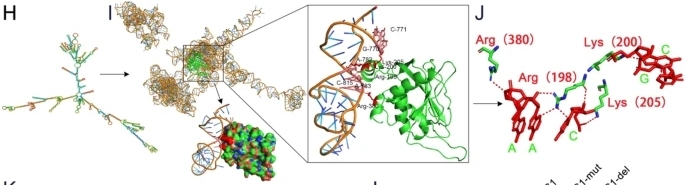 Fig. 1 Structural Basis of APCDD1L-AS1 Interaction with DLST.1
Fig. 1 Structural Basis of APCDD1L-AS1 Interaction with DLST.1
Key structural properties of DLST:
- Classical E2 enzyme triple domain folding pattern
- Flexible lysine linker arm with lipoyl binding site
- Conserved histidine - glutamic acid catalytic diad
- Substrate specific hydrophobic pockets maintain the stability of the enzyme active center
Functions of DLST
The core function of the DLST gene-encoded protein is to serve as a key component of the ketoglutarate dehydrogenase complex. It mainly participates in the rate-limiting step of the tricarboxylic acid cycle by catalyzing the succinylation reaction of the thioacyl group.
| Function | Description |
| Regulation of energy metabolism | Catalyze the conversion of α -ketoglutaric acid to succinyl-coA, generating NADH and ATP precursors to maintain cellular energy homeostasis. |
| Metabolite channel effect | Through the synergistic effect between domains, the efficient transfer of substrates within the enzyme complex is achieved, and the diffusion of intermediate products is avoided. |
| Oxidative stress response | Regulate the generation rate of reactive oxygen species precursors and affect the mitochondrial REDOX balance state. |
| Lipid synthesis support | For fatty acid synthesis to provide the necessary succinyl coa substrates, connection of sugar metabolism and lipid metabolic pathways. |
| Tumor metabolic reprogramming | Aberrant expression in a variety of cancer cells promotes the Warburg effect by changing the flux of the tricarboxylic acid cycle. |
The catalytic kinetics of this enzyme exhibits typical Mie equation characteristics, and its activity is regulated at multiple levels by substrate concentration, product feedback inhibition and phosphorylation modification, reflecting the precise control mechanism of metabolic hub nodes.
Applications of DLST and DLST Antibody in Literature
1. Shen, Ning, et al. "DLST-dependence dictates metabolic heterogeneity in TCA-cycle usage among triple-negative breast cancer." Communications biology 4.1 (2021): 1289. https://doi.org/10.1038/s42003-021-02805-8
The article indicates that in triple-negative breast cancer, high expression of DLST predicts a poor prognosis for patients. Research has found that inhibiting DLST can specifically suppress the growth of some TNBC cells and promote their death by influencing the tricarboxylic acid cycle and reactive oxygen species pathways, providing a potential therapeutic target for this subtype.
2. Kuhn, Christina, et al. "Candidate drugs associated with sensitivity of cancer cell lines with DLST amplification or high mRNA levels." Oncotarget 14 (2023): 14. https://doi.org/10.18632/oncotarget.28342
Studies have shown that cancer cells with high expression or amplification of DLST are more sensitive to drugs targeting specific pathways. In addition to the known OXPHOS inhibitors, these cells also demonstrated sensitivity to BCL2 inhibitors and multiple ERK/MAPK pathway kinase inhibitors, providing a new potential therapeutic direction for DLST-abnormal tumors.
3. Yang, Rongjin, et al. "Grpel2 maintains cardiomyocyte survival in diabetic cardiomyopathy through DLST-mediated mitochondrial dysfunction: a proof-of-concept study." Journal of translational medicine 21.1 (2023): 200. https://doi.org/10.1186/s12967-023-04049-y
This study reveals for the first time that in diabetic cardiomyopathy, Grpel2 positively regulates the process of its input into mitochondria by interacting with DLST. Overexpression of Grpel2 can improve mitochondrial dysfunction and apoptosis by maintaining DLST function, indicating that it can serve as a potential therapeutic target.
4. Zhang, Quanli, et al. "Hypoxia-inducible APCDD1L-AS1 promotes osimertinib resistance by stabilising DLST to drive tricarboxylic acid cycle in lung adenocarcinoma." Journal of Experimental & Clinical Cancer Research 44.1 (2025): 197. https://doi.org/10.1186/s13046-025-03462-z
This study reveals that in osimertinib-resistant lung adenocarcinoma, hypoxia-induced APCDD1L-AS1 promotes drug resistance by stabilizing the DLST protein and accelerating the tricarboxylic acid cycle. The HIF-1α/APCDD1L-AS1/DLST axis provides a new potential therapeutic target for overcoming drug resistance.
5. Li, Chang, et al. "Case report: A rare DLST mutation in patient with metastatic pheochromocytoma: clinical implications and management challenges." Frontiers in Oncology 14 (2024): 1394552. https://doi.org/10.3389/fonc.2024.1394552
This case report identified a patient with pheochromocytoma carrying a rare germline mutation of the DLST gene. This case presented with multiple metastases after the operation and had a limited response to chemotherapy and other treatments, suggesting that DLST mutations may be associated with the invasiveness and poor prognosis of pheochromocytoma.
Creative Biolabs: DLST Antibodies for Research
Creative Biolabs specializes in the production of high-quality DLST antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom DLST Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our DLST antibodies, custom preparations, or technical support, contact us at email.
Reference
- Zhang, Quanli, et al. "Hypoxia-inducible APCDD1L-AS1 promotes osimertinib resistance by stabilising DLST to drive tricarboxylic acid cycle in lung adenocarcinoma." Journal of Experimental & Clinical Cancer Research 44.1 (2025): 197. https://doi.org/10.1186/s13046-025-03462-z
Anti-DLST antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CD247 Recombinant Antibody (6B10.2) (CBMAB-C1583-YY)

-
Mouse Anti-2C TCR Recombinant Antibody (V2-1556) (CBMAB-0951-LY)

-
Mouse Anti-CCDC25 Recombinant Antibody (CBLC132-LY) (CBMAB-C9786-LY)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Mouse Anti-ABIN2 Recombinant Antibody (V2-179106) (CBMAB-A0349-YC)

-
Mouse Anti-COL1A2 Recombinant Antibody (CF108) (V2LY-1206-LY626)

-
Mouse Anti-BLNK Recombinant Antibody (CBYY-0623) (CBMAB-0626-YY)

-
Mouse Anti-DMPK Recombinant Antibody (CBYCD-324) (CBMAB-D1200-YC)

-
Rabbit Anti-B2M Recombinant Antibody (CBYY-0059) (CBMAB-0059-YY)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Mouse Anti-BACE1 Recombinant Antibody (CBLNB-121) (CBMAB-1180-CN)

-
Mouse Anti-ADV Recombinant Antibody (V2-503423) (CBMAB-V208-1364-FY)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503417) (CBMAB-V208-1369-FY)

-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-BIRC3 Recombinant Antibody (16E63) (CBMAB-C3367-LY)

-
Mouse Anti-BLK Recombinant Antibody (CBYY-0618) (CBMAB-0621-YY)

-
Mouse Anti-FOXA3 Recombinant Antibody (2A9) (CBMAB-0377-YC)

-
Mouse Anti-CDKL5 Recombinant Antibody (CBFYC-1629) (CBMAB-C1689-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






