EPHA2 Antibodies
Background
The EPHA2 gene encodes a transmembrane protein belonging to the tyrosine kinase receptor family, which is widely expressed in epithelial cells and tissues. It regulates key biological processes such as cell migration, adhesion and morphogenesis by binding to its ligand ephrin-A and mediating the rejection signal transduction between cells. This gene plays a significant role in embryonic development, tissue boundary formation and angiogenesis, and its abnormal function is closely related to the proliferation, invasion and metastasis of various cancers. EPHA2 was first identified in 1990. The analysis of its structure revealed the specific mechanism of receptor-ligand interaction, providing an important foundation for targeted therapy research. In-depth research on this gene signal continuously drives the development of fields such as cell communication and tumorigenesis mechanisms.
Structure of EPHA2
EPHA2 is a type I transmembrane protein with a molecular weight of approximately 130 kDa. The molecular weight of this protein varies among different species, mainly due to the subtle changes in the glycosylation modification degree of its extracellular domain and the amino acid sequence.
| Species | Human | Mouse | Rat | Chicken | Zebrafish |
| Molecular Weight (kDa) | 108-130 | 107-128 | 109-129 | 105-125 | ~110 |
| Primary Structural Differences | Contains a fibronectin III domain and two calcium-dependent domains | Highly homologous to the human sequence, the intracellular tyrosine kinase domain is conserved | Extracellular region has a unique glycosylation sites | Lack of an intact intracellular regulatory motif | Shorter extracellular domain structure, function is relatively primitive |
EPHA2 is composed of 975 amino acids. Its structure includes an extracellular region (consisting of a ligand-binding domain, a fibronectin III repeat sequence, and two calcium-dependent domains), a transmembrane region, and an intracellular tyrosine kinase domain. The ligand binding mechanism of this protein depends on the global conformational changes in its extracellular region: when binding to the ephrin-A ligand, the two EPHA2 molecules form a dimer, initiating the autophosphylation of specific residues (such as Y594 and Y772) in the tyrosine kinase domain, thereby initiating downstream signal transduction. This precise conformational regulation mechanism ensures that EPHA2 can play a crucial guiding role in processes such as cell communication, tissue boundary formation, and cell migration.
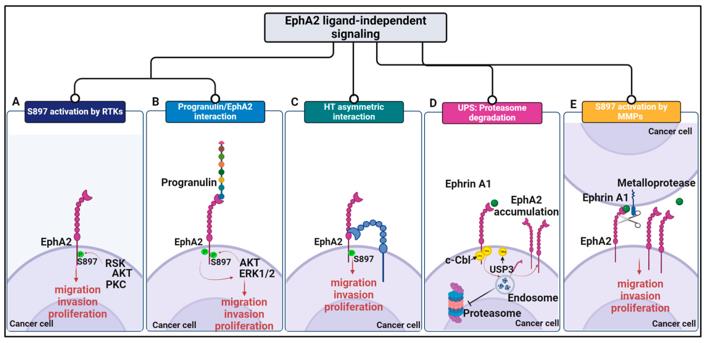 Fig. 1 Schematic representation of EphA2-driven mechanisms in cancer.1
Fig. 1 Schematic representation of EphA2-driven mechanisms in cancer.1
Key structural properties of EPHA2:
- The extracellular region contains ligand-binding domains and fibronectin repeats
- A single transmembrane helix is anchored to the cell membrane
- Intracellular parts as the typical tyrosine kinase catalytic domain structure
- Carboxyl terminal of hydrophobic amino acids regulate its phosphorylation activity
Functions of EPHA2
The EPHA2 receptor plays a core role in cell communication and tissue construction, and its function shows significant context dependence: it guides cell localization in normal tissues, while promoting malignant progression in pathological conditions.
| Function | Description |
| Cell rejection and boundary formation | After ephrin-A activation, rejection signals are transmitted, guiding cell migration pathways and establishing tissue boundaries, which is particularly crucial in neural tube and vascular development. |
| Contact inhibition regulation | In normal epithelial cells, the high expression of EPHA2 inhibits the Ras-MAPK pathway, limits excessive cell proliferation, and maintains tissue homeostasis. |
| Tumor promotion and invasion | In various cancer tissues, EPHA2 is often overexpressed and loses ligand-dependent activation, and instead drives cell proliferation, invasion and metastasis through the AKT-mTOR pathway. |
| Angiogenesis regulation | By regulating the VEGF signaling pathway and the behavior of vascular endothelial cells, it affects tumor angiogenesis and provides blood supply for rapidly growing tumors. |
| Potential of drug targets | The functions under different physiological pathology duality, makes it the targeted cancer therapy, especially of inhibiting metastasis potential targets. |
The signal output of EPHA2 is highly dependent on its tyrosine phosphorylation status: ligand activation triggers auto-phosphorylation and initiates inhibitory signals, while non-ligand activation leads to abnormal phosphorylation patterns, which in turn drive cancer-promoting signals. This bidirectional regulatory mechanism explains the functional differences between EPHA2 in development and disease.
Applications of EPHA2 and EPHA2 Antibody in Literature
1. Toracchio, Lisa, et al. "EphA2 in cancer: molecular complexity and therapeutic opportunities." International Journal of Molecular Sciences 25.22 (2024): 12191. https://doi.org/10.3390/ijms252212191
The article indicates that the oncoprotein EphA2 has a complex function and drives tumor deterioration. Limited by traditional therapies, new strategies based on their molecular mechanisms, such as immunotherapy and PROTAC degraders, have shown great therapeutic potential.
2. Xiao, Ta, et al. "Targeting EphA2 in cancer." Journal of hematology & oncology 13.1 (2020): 114. https://doi.org/10.1186/s13045-020-00944-9
The article indicates that EphA2 is highly expressed in tumors and is a potential therapeutic target. Its signaling axis with the ligand ephrin A1 promotes carcinogenesis and drug resistance. Although the clinical efficacy of existing targeted drugs is limited, it remains an important research and development direction for anti-cancer strategies.
3. Gai, Qu-Jing, et al. "EPHA2 mediates PDGFA activity and functions together with PDGFRA as prognostic marker and therapeutic target in glioblastoma." Signal transduction and targeted therapy 7.1 (2022): 33. https://doi.org/10.1038/s41392-021-00855-2
This study reveals that EPHA2 is a new potential receptor for PDGFA, and the combination of the two can independently activate cancer-promoting signals. Simultaneous inhibition of EPHA2 and PDGFRA can synergistically prevent the growth of glioma, overcome single-drug resistance, and provide a new treatment strategy.
4. El Fakiri, Mohamed, et al. "Development and preclinical characterization of a novel radiotheranostic EphA2-targeting bicyclic peptide." Theranostics 14.12 (2024): 4701. https://doi.org/10.7150/thno.96641
This study developed a novel bicyclic peptide, BCY18469, as an EPHA2-targeted radioactive diagnostic and therapeutic agent. It has demonstrated excellent properties such as high affinity, specific tumor enrichment and rapid renal clearance in preclinical studies, indicating the great potential of bicyclic peptides in diagnosis and treatment applications.
5. Giordano, Giorgia, et al. "Targeting the EphA2 pathway: could it be the way for bone sarcomas?." Cell Communication and Signaling 22.1 (2024): 433. https://doi.org/10.1186/s12964-024-01811-7
This study indicates that EphA2 is overexpressed in osteosarcoma and is a key oncoprotein mediating metastasis and chemotherapy resistance. For unresectable advanced patients, targeting EphA2 provides a new strategy for the precise diagnosis and treatment of osteosarcoma, chondrosarcoma and Ewing's sarcoma.
Creative Biolabs: EPHA2 Antibodies for Research
Creative Biolabs specializes in the production of high-quality EPHA2 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom EPHA2 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our EPHA2 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Toracchio, Lisa, et al. "EphA2 in cancer: molecular complexity and therapeutic opportunities." International Journal of Molecular Sciences 25.22 (2024): 12191. https://doi.org/10.3390/ijms252212191
Anti-EPHA2 antibodies
 Loading...
Loading...
Hot products 
-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

-
Mouse Anti-AGK Recombinant Antibody (V2-258056) (CBMAB-M0989-FY)

-
Mouse Anti-EGR1 Recombinant Antibody (CBWJZ-100) (CBMAB-Z0289-WJ)

-
Mouse Anti-CAT Recombinant Antibody (724810) (CBMAB-C8431-LY)

-
Mouse Anti-CCDC6 Recombinant Antibody (CBXC-0106) (CBMAB-C5397-CQ)

-
Mouse Anti-AAV8 Recombinant Antibody (V2-634028) (CBMAB-AP022LY)

-
Mouse Anti-CCNH Recombinant Antibody (CBFYC-1054) (CBMAB-C1111-FY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Mouse Anti-DMPK Recombinant Antibody (CBYCD-324) (CBMAB-D1200-YC)

-
Mouse Anti-BIRC3 Recombinant Antibody (16E63) (CBMAB-C3367-LY)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Mouse Anti-ACTN4 Recombinant Antibody (V2-6075) (CBMAB-0020CQ)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-Acetyl-α-Tubulin (Lys40) Recombinant Antibody (V2-623485) (CBMAB-CP2897-LY)

-
Mouse Anti-CTCF Recombinant Antibody (CBFYC-2371) (CBMAB-C2443-FY)

-
Rabbit Anti-ALOX5AP Recombinant Antibody (CBXF-1219) (CBMAB-F0750-CQ)

-
Mouse Anti-8-oxoguanine Recombinant Antibody (V2-7719) (CBMAB-1898CQ)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Mouse Anti-FOSB Recombinant Antibody (CBXF-3593) (CBMAB-F2522-CQ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






