FAAH Antibodies
Background
FAAH is an endogenous hydrolase located in cell membranes, mainly distributed in the central nervous system, liver and immune tissues of mammals. This enzyme participates in regulating physiological processes such as neurotransmitter release, pain perception and inflammatory response by catalyzing the degradation of endogenous cannabinoids (such as ethanolamine arachidonic acid) and other fatty acid amide signaling molecules. Since its first identification by the Cravatt team in 1996, FAAH has become an important research subject in the fields of neuropharmacology and pain treatment due to its crucial role in terminating endogenous cannabinoid signaling. The analysis of its crystal structure not only reveals the catalytic mechanism characteristics of the serine hydrolase family, but also provides a molecular basis for the development of targeted inhibitors for anxiety, chronic pain and related mental disorders, promoting the progress in the field of precision medicine.
Structure of FAAH
FAAH is a transmembrane protein of approximately 63 kDa. Its molecular weight varies slightly among different species, mainly due to the evolutionary adaptability of amino acid sequences.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | About 63 | About 62 | About 63 |
| Primary Structural Differences | With serine - glycine catalytic dual body | Highly conserved structure of catalytic domain | The homology with human FAAH is extremely high |
This protein is composed of approximately 579 amino acids, and its tertiary structure exhibits a typical α/β hydrolase folding. The active center contains a unique "transmembrane channel" structure that is responsible for guiding lipid substrates into the catalytic core. The serine-ribonucrenase family characteristic sequence (Ser-Ser-Lys) in the catalytic chamber constitutes the key site of the hydrolysis reaction, while the phobic domain ensures effective anchoring to the cell membrane. This ingenious spatial arrangement enables FAAH to specifically recognize and cleave the amide bonds of endogenous cannabinoid molecules.
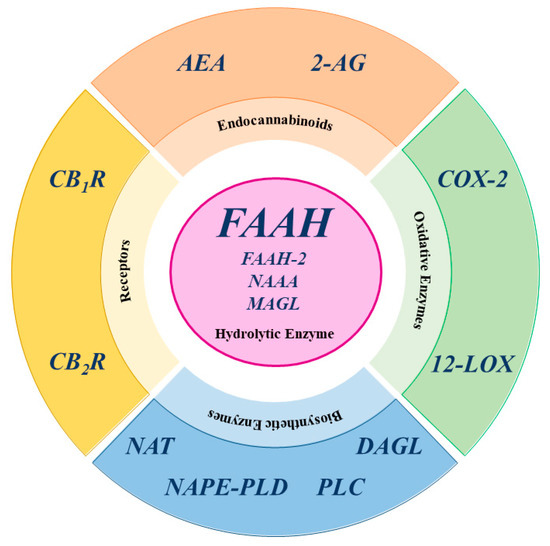 Fig. 1 The Central Role of FAAH in the Endocannabinoid System.1
Fig. 1 The Central Role of FAAH in the Endocannabinoid System.1
Key structural properties of FAAH:
- Typical α/β hydrolase folding configuration
- Lead the substrate into unique across the membrane channel
- Serine-nucleophilic attack sequence of the catalytic center
Functions of FAAH
The core function of FAAH is to regulate the endogenous cannabinoid signaling pathway. However, it is also widely involved in the regulation of various pathophysiological processes, including pain perception, emotional stability and inflammatory response.
| Function | Description |
| Signal termination | By hydrolyzing endogenous cannabinoids such as ethanolamine arachidonic acid, the synaptic retrograde signal transmission mediated by them is promptly terminated. |
| Pain regulation | Degradation in the central and peripheral nervous system pain cannabinoid, thereby directly regulate and persistent pain signals. |
| Emotion regulation | In the limbic system, it influences the manifestation of emotion-related behaviors such as anxiety and depression by regulating cannabinoid levels. |
| Inflammation suppression | By degrading cannabinoid substances with immune regulatory functions, it indirectly affects the release of inflammatory factors and the activity of immune cells. |
| Neuroprotection | Maintain the homeostasis of cannabinoid signals in the nervous system, avoid excessive excitatory toxicity, and thereby exert a protective effect. |
The enzymatic activity of FAAH shows a typical substrate concentration-dependent pattern, with its hydrolysis efficiency monotonically increasing as the substrate concentration rises. This is in stark contrast to the S-shaped kinetic curve of allosteric enzymes, reflecting its role as a "precise switch" for signaling pathways.
Applications of FAAH and FAAH Antibody in Literature
1. Mallet, Christophe, Claude Dubray, and Christian Dualé. "FAAH inhibitors in the limelight, but regrettably." International journal of clinical pharmacology and therapeutics 54.7 (2016): 498. https://doi.org/10.5414/CP202687
This article summarizes the research and development progress of the FAAH inhibitor BIA 10-2474, with a focus on the serious neurotoxic adverse events it caused in Phase I clinical trials. This incident led to one brain death and five hospitalizations, raising significant safety concerns in the development of FAAH inhibitors. The cause of the accident is still under investigation at present.
2. Mikaeili, Hajar, et al. "Molecular basis of FAAH-OUT-associated human pain insensitivity." Brain 146.9 (2023): 3851-3865. https://doi.org/10.1093/brain/awad098
This article summarizes that the newly discovered long non-coding RNA gene FAAH-out can regulate the expression of the FAAH gene. The mechanism includes influencing the methylation of the FAAH promoter and the effect of enhancers, which provides a brand-new target and theoretical basis for the development of new therapies for pain and anxiety.
3. Nicoara, Catalin, Filomena Fezza, and Mauro Maccarrone. "FAAH Modulators from Natural Sources: A Collection of New Potential Drugs." Cells 14.7 (2025): 551. https://doi.org/10.3390/cells14070551
This article Outlines that natural substances (such as plant extracts and flavonoids) can inhibit fatty acid amide hydrolase (FAAH), thereby increasing endogenous cannabinoid levels. These natural inhibitors, due to their structural diversity and potential low toxicity, offer broad prospects for the development of new drugs for treating related diseases.
4. Tripathy, Mallika, et al. "FAAH inhibition ameliorates breast cancer in a murine model." Oncotarget 14 (2023): 910. https://doi.org/10.18632/oncotarget.28534
Studies have shown that inhibiting fatty acid amide hydrolase (FAAH) in breast cancer cells, especially when used in combination with endogenous cannabinoids, can effectively induce apoptosis of cancer cells and inhibit tumor growth. This provides a new alternative strategy to chemotherapy for breast cancer treatment and has a promising future.
5. Della Pietra, Adriana, et al. "Potent dual MAGL/FAAH inhibitor AKU-005 engages endocannabinoids to diminish meningeal nociception implicated in migraine pain." The Journal of Headache and Pain 24.1 (2023): 38. https://doi.org/10.1186/s10194-023-01568-3
Studies have shown that in the treatment of migraines, inhibiting FAAH and MAGL can significantly increase the level of endocannabinoids. The novel dual inhibitor AKU-005 effectively inhibits meningial nosgenic signals by activating the CB1 receptor, demonstrating the potential of an alternative analgesic therapy.
Creative Biolabs: FAAH Antibodies for Research
Creative Biolabs specializes in the production of high-quality FAAH antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom FAAH Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our FAAH antibodies, custom preparations, or technical support, contact us at email.
Reference
- Nicoara, Catalin, Filomena Fezza, and Mauro Maccarrone. "FAAH Modulators from Natural Sources: A Collection of New Potential Drugs." Cells 14.7 (2025): 551. https://doi.org/10.3390/cells14070551
Anti-FAAH antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-AMH Recombinant Antibody (5/6) (CBMAB-A2527-YC)

-
Mouse Anti-EMP3 Recombinant Antibody (CBFYE-0100) (CBMAB-E0207-FY)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-1728) (CBMAB-2077-YY)

-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

-
Mouse Anti-CD59 Recombinant Antibody (CBXC-2097) (CBMAB-C4421-CQ)

-
Mouse Anti-AKR1B1 Antibody (V2-2449) (CBMAB-1001CQ)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-CRYAB Recombinant Antibody (A4345) (CBMAB-A4345-YC)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

-
Rabbit Anti-BAD (Phospho-Ser136) Recombinant Antibody (CAP219) (CBMAB-AP536LY)

-
Mouse Anti-ASB9 Recombinant Antibody (1D8) (CBMAB-A0529-LY)

-
Mouse Anti-ADRB2 Recombinant Antibody (V2-180026) (CBMAB-A1420-YC)

-
Mouse Anti-CD2AP Recombinant Antibody (BR083) (CBMAB-BR083LY)

-
Rabbit Anti-CAMK2A Recombinant Antibody (BA0032) (CBMAB-0137CQ)

-
Mouse Anti-ALB Recombinant Antibody (V2-55272) (CBMAB-H0819-FY)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

-
Mouse Anti-EGR1 Recombinant Antibody (CBWJZ-100) (CBMAB-Z0289-WJ)

-
Mouse Anti-ARSA Recombinant Antibody (CBYC-A799) (CBMAB-A3679-YC)

-
Rat Anti-C5AR1 Recombinant Antibody (8D6) (CBMAB-C9139-LY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








