GAMT Antibodies
Background
GAMT is a metabolic enzyme mainly distributed in the liver and kidneys of vertebrates. This enzyme catalyzes the methylation reaction of sarcosine to generate creatine, providing a molecular basis for the storage and transportation of high-energy phosphates in the body. Because the energy metabolism of brain tissue and muscle tissue is highly dependent on the creatine phosphate system, the deficiency of GAMT function will directly lead to neurodevelopmental disorders and muscle weakness symptoms. This gene was first identified in 1994, and the crystal structure of the protein it encodes was analyzed in the early 21st century. It not only revealed the catalytic mechanism of S-adenosylmethionine-dependent methyltransferase, but also provided a target for the diagnosis and treatment of hereditary creatine deficiency syndrome. As a key molecule in the energy metabolism pathway, GAMT continuously provides theoretical models for research on genetic disease treatment strategies and cellular energy regulation.
Structure of GAMT
GAMT is a metabolic enzyme with a molecular weight of approximately 26 kDa. There are minor differences in its molecular weight among different species, mainly due to variations in amino acid sequences.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 26.0 | 25.8 | 25.9 | 26.1 |
| Primary Structural Differences | Highly conserved structure of catalytic domain | It has more than 90% homology with humans | Active site amino acids | There are individual neutral amino acid substitutions |
This protein is composed of approximately 240 amino acids and folds to form a typical spatial structure of methyltransferase. Its core is an S-adenosylmethionine binding domain composed of mixed β -sheets, surrounded by multiple α -helices. Two key acidic amino acids in the active center are responsible for anchoring the substrate creatine, while a conserved aromatic amino acid ring fixes the methyl donor through hydrophobic interaction, jointly completing the transmethylation catalytic reaction.
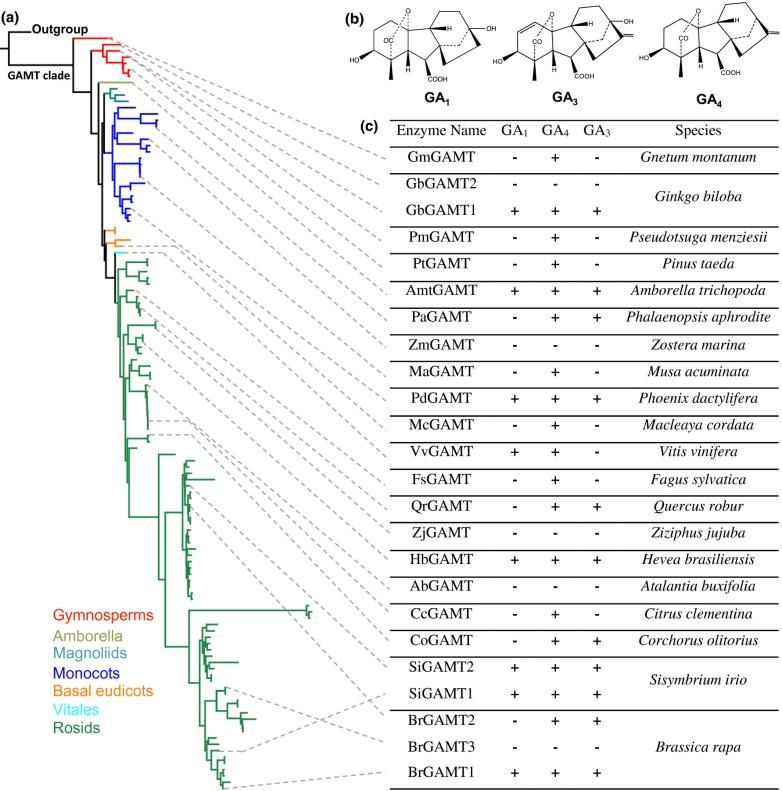 Fig. 1 GAMT clade and biochemical activities.1
Fig. 1 GAMT clade and biochemical activities.1
Key structural properties of GAMT:
- Typical methyltransferase folding conformation
- The core substrate binding pocket composed of β sheets
- Conservative S - adenosine methionine domain is responsible for the catalytic methylation
Functions of GAMT
The core function of the GAMT gene is to catalyze creatine synthesis. However, this enzyme is also involved in various physiological and pathological processes, including the maintenance of energy homeostasis and the development of the nervous system.
| Function | Description |
| Creatine synthesis | In the liver, it catalyzes the transmethylation reaction between creatine and S-adenosylmethionine to generate creatine precursors. |
| Energy buffer | By maintaining creatine phosphate levels, it provides immediate ATP buffering for high-energy-consuming tissues such as the brain and muscles. |
| Nervous system protection | Ensuring the energy supply of the central nervous system, its functional defects directly lead to intellectual retardation and epilepsy. |
| Metabolite regulation | Effectively reduce the accumulation of creatine toxicity in the body and simultaneously control the level of S-adenosine homocysteine by-products. |
| Sports endurance support | Maintaining the recycling efficiency of high-energy phosphates in muscles is crucial for the energy supply during sustained physical activity. |
The catalytic efficiency of this enzyme is regulated by the level of S-adenosylmethionine. This substrate-dependent regulatory mechanism makes it a key node connecting the methionine cycle with cellular energy metabolism.
Applications of GAMT and GAMT Antibody in Literature
1. Rossi, Luigia, et al. "Intellectual disability and brain creatine deficit: phenotyping of the genetic mouse model for GAMT deficiency." Genes 12.8 (2021): 1201. https://doi.org/10.3390/genes12081201
The article indicates that GAMT gene defects lead to GAMT deficiency, causing creatine deficiency and guanidine acetic acid accumulation in the brain. The clinical manifestations of this disease include intellectual disability, ataxia and epilepsy. However, GAMT gene knockout mice only show mild behavioral abnormalities, and the neurochemical and molecular mechanisms still need to be further studied.
2. Yuan, Hao, et al. "Role of a novel circRNA-CGNL1 in regulating pancreatic cancer progression via NUDT4–HDAC4–RUNX2–GAMT-mediated apoptosis." Molecular Cancer 23.1 (2024): 27. https://doi.org/10.1186/s12943-023-01923-7
Studies have revealed that the circular RNA circCGNL1 upregulates the expression of the tumor suppressor gene GAMT in pancreatic cancer through the NUDT4/HDAC4 signaling axis. GAMT can activate the AMPK-AKT-BAD pathway, thereby inducing apoptosis of cancer cells and inhibiting tumor growth. This discovery provides a new potential target for the treatment of pancreatic cancer.
3. Zhang, Chi, et al. "Origin and evolution of a gibberellin‐deactivating enzyme GAMT." Plant direct 4.12 (2020): e00287. https://doi.org/10.1002/pld3.287
This study has for the first time discovered that gibberellin methyltransferase (GAMT) is a gene specific to seed plants, whose function is to inactivate active gibberellin. GAMT is highly expressed in flowers and seeds, and its function has been conserved during evolution. However, more than two-thirds of angiosperms have lost this gene, and its retention or loss pattern suggests its special significance in the adaptive evolution of plants.
4. Oosthoek, Ezra D., et al. "Gender-affirming medical treatment for adolescents: a critical reflection on "effective" treatment outcomes." BMC Medical Ethics 25.1 (2024): 1-20. https://doi.org/10.1186/s12910-024-01143-8
Most existing studies define the "effectiveness" of adolescent GAMT through "condition improvement", but this criterion implies normative assumptions about gender identity. This article critically reflects on this linear narrative, emphasizing the need to clarify treatment goals and give transgender adolescents a core say in the evaluation of therapeutic effects.
5. Rosko, Lauren M., et al. "Cerebral creatine deficiency affects the timing of oligodendrocyte myelination." Journal of Neuroscience 43.7 (2023): 1143-1153. https://doi.org/10.1523/JNEUROSCI.2120-21.2022
This study reveals that the enzyme GAMT is the key to endogenous creatine synthesis in the brain. GAMT is specifically expressed in oligodendrocytes during the myelin formation period. Its absence can lead to delayed myelin development and axonal injury. Supplementation of creatine can promote myelin regeneration, indicating that GAMT plays a core role in nervous system development by regulating cellular energy metabolism.
Creative Biolabs: GAMT Antibodies for Research
Creative Biolabs specializes in the production of high-quality GAMT antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom GAMT Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our GAMT antibodies, custom preparations, or technical support, contact us at email.
Reference
- Zhang, Chi, et al. "Origin and evolution of a gibberellin‐deactivating enzyme GAMT." Plant direct 4.12 (2020): e00287. https://doi.org/10.1002/pld3.287
Anti-GAMT antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-BZLF1 Recombinant Antibody (BZ.1) (CBMAB-AP705LY)

-
Mouse Anti-CDKL5 Recombinant Antibody (CBFYC-1629) (CBMAB-C1689-FY)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

-
Rabbit Anti-ALK (Phosphorylated Y1278) Recombinant Antibody (D59G10) (PTM-CBMAB-0035YC)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

-
Rabbit Anti-BRCA2 Recombinant Antibody (D9S6V) (CBMAB-CP0017-LY)

-
Mouse Anti-DMD Recombinant Antibody (D1190) (CBMAB-D1190-YC)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Rabbit Anti-ENO2 Recombinant Antibody (BA0013) (CBMAB-0272CQ)

-
Rat Anti-4-1BB Recombinant Antibody (V2-1558) (CBMAB-0953-LY)

-
Rabbit Anti-ABL1 (Phosphorylated Y245) Recombinant Antibody (V2-505716) (PTM-CBMAB-0465LY)

-
Mouse Anti-AHCYL1 Recombinant Antibody (V2-180270) (CBMAB-A1703-YC)

-
Mouse Anti-AKT1 Recombinant Antibody (V2-180546) (CBMAB-A2070-YC)

-
Mouse Anti-C4B Recombinant Antibody (CBYY-C2996) (CBMAB-C4439-YY)

-
Mouse Anti-CD247 Recombinant Antibody (6B10.2) (CBMAB-C1583-YY)

-
Rat Anti-ADAM10 Recombinant Antibody (V2-179741) (CBMAB-A1103-YC)

-
Mouse Anti-ADGRL2 Recombinant Antibody (V2-58519) (CBMAB-L0166-YJ)

-
Mouse Anti-8-oxoguanine Recombinant Antibody (V2-7719) (CBMAB-1898CQ)

-
Rabbit Anti-ALDOA Recombinant Antibody (D73H4) (CBMAB-A2314-YC)

-
Mouse Anti-BIRC3 Recombinant Antibody (315304) (CBMAB-1214-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








