HEYL Antibodies
Background
The HEYL protein encoded by the HEYL gene is a member of the bHLH transcription factors in the HES-related family and mainly acts as an effector factor of the Notch signaling pathway, playing a regulatory role in the development of various tissues and organs. This protein participates in key biological processes such as cell differentiation, tissue pattern establishment, and stem cell maintenance by binding to the specific DNA sequence box E and inhibiting the transcription of downstream target genes. During embryonic development, the expression of HEYL is particularly important for the normal morphogenesis of the cardiovascular system, nervous system and kidneys, and its functional abnormalities have been confirmed to be associated with a variety of congenital defects and diseases. Since its identification in the early 21st century, researchers have gradually revealed the core position of HEYL in the developmental regulatory network through gene knockout models and molecular biology techniques, providing an important theoretical basis for understanding transcriptional suppression mechanisms and cell fate determination.
Structure of HEYL
The HEYL protein is a transcription factor with a molecular weight of approximately 30-35 kDa. This protein belongs to the bHLH-Orange superfamily. Its molecular weight is relatively conserved among different mammals, and the main difference lies in the amino acid substitution in the non-domain region.
| Species | Human | Mouse | Rat | Bovine |
| Molecular Weight (kDa) | 33.2 | 33.1 | 33.3 | 33.0 |
| Primary Structural Differences | Two key domain structure containing bHLH and Orange | The bHLH domain is highly conserved | There is a single amino acid variation in the Orange domain | The similarity to the human sequence reaches 95% |
The HEYL protein is composed of approximately 300 amino acids. The core of its three-dimensional structure is the bHLH domain (basic helical-ring-helical), which forms a typical helical-rotation-helical conformation and is responsible for DNA binding and dimerization. The adjacent Orange domain presents a special cyclic conformation that can stabilize protein-protein interactions. These two domains together form the functional core of HEYL: the bHLH region recognizes and binds to the E-box sequence (CANNTG), while the Orange domain mediates interactions with other transcriptional co-repressors, thereby achieving precise regulation of gene expression.
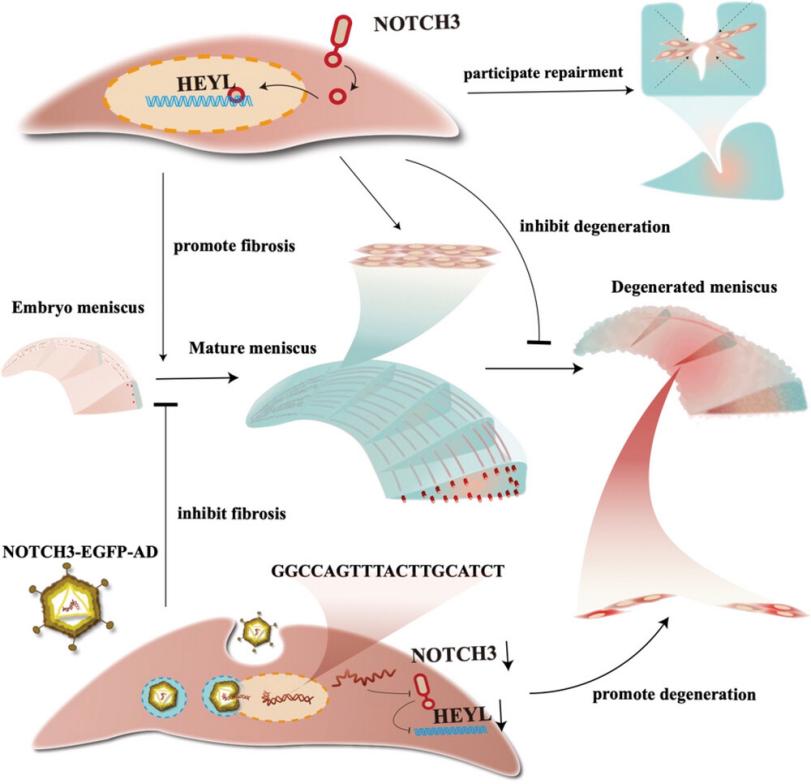 Fig. 1 HEYL mediates NOTCH3-driven meniscus fibrosis.1
Fig. 1 HEYL mediates NOTCH3-driven meniscus fibrosis.1
Key structural properties of HEYL:
- The conserved bHLH (basic helix-loop-helix) domain is responsible for DNA recognition and binding
- Orange structure domain specificity protein interaction interface
- Basic amino acid area mediates the specificity of the target genes E box sequence identification
- Spiral - ring - helical conformation to inhibit transcription complex assembly structure
Functions of HEYL
The core function of the HEYL gene is to act as a transcriptional suppressor of the Notch signaling pathway and participate in cell fate determination. Meanwhile, this gene plays a regulatory role in a variety of physiological and pathological processes.
| Function | Description |
| Transcriptional inhibition | By binding to the E-box sequence, the transcriptional activity of downstream target genes is inhibited. |
| Regulation of cell differentiation | Prevent premature cell differentiation in various tissues and organs and maintain the pool of progenitor cells. |
| Formation of developmental patterns | Participate in the process of boundary establishment and morphogenesis in organ development. |
| Disease association | The abnormal expression is closely related to a variety of cancer development. |
| Signal path integration | Coordinate the interaction between Notch signaling and other developmental pathways. |
HEYL and its family members HES/HEY have both overlapping and specific functions: compared with HES protein which mainly binds to n-box sequences, HEYL specifically recognizes E-box and has a stronger transcriptional inhibitory ability. This functional differentiation enables HEYL to play an irreplaceable regulatory role in specific developmental stages and tissues.
Applications of HEYL and HEYL Antibody in Literature
1. Han, Liangfeng, et al. "HEYL regulates neoangiogenesis through overexpression in both breast tumor epithelium and endothelium." Frontiers in Oncology 10 (2021): 581459. https://doi.org/10.3389/fonc.2020.581459
Research has found that the HEYL gene promotes angiogenesis and progression of breast cancer by directly up-regulating factors such as CXCL1/2/3. Inhibiting HEYL or its downstream signals can effectively suppress tumor blood vessels and growth, suggesting it as a new target for anti-vascular therapy.
2. Fadel, Hewida H., et al. "Partial‐Methylated HeyL Promoter Predicts the Severe Illness in Egyptian COVID‐19 Patients." Disease Markers 2022.1 (2022): 6780710. https://doi.org/10.1155/2022/6780710
Studies have found that the HeyL gene promoter in Egyptian COVID-19 patients is in a unique "hypomethylation" state, which may enhance its gene activity and be associated with severe symptoms such as fever and pneumonia. The methylation status of HeyL may serve as a prognostic marker.
3. Lin, Qimei, et al. "The HeyL-aromatase axis promotes cancer stem cell properties by endogenous estrogen-induced autophagy in castration-resistant prostate cancer." Frontiers in Oncology 11 (2022): 787953. https://doi.org/10.3389/fonc.2021.787953
Research has found that HEYL directly activates the CYP19A1 gene in castration-resistant prostate cancer, increasing the levels of aromatase and endogenous estrogen, thereby promoting the characteristics and drug resistance of cancer stem cells. Targeting this pathway may become a new therapeutic strategy.
4. Bodas, Manish, et al. "The NOTCH3 downstream target HEYL is required for efficient human airway basal cell differentiation." Cells 10.11 (2021): 3215. https://doi.org/10.3390/cells10113215
Research has found that HEYL, a downstream target of NOTCH3 signaling, is a key factor in regulating the differentiation of airway basal cells. Knockdown of HEYL can damage the differentiation of various epithelial cells such as goblet cells and ciliated cells, while overexpression of HEYL in COPD cells can reverse their differentiation disorders.
5. Brahim, Sonia, et al. "Notch3 regulates Mybl2 via HeyL to limit proliferation and tumor initiation in breast cancer." Cell Death & Disease 14.2 (2023): 171. https://doi.org/10.1038/s41419-023-05674-7
Research has found that Notch3 inhibits the cell cycle regulator Mybl2 through its downstream target HEYL, thereby delaying the occurrence of breast cancer. Notch3 deletion accelerates tumor formation, revealing the protective role of the Notch3-Heyl pathway in breast cancer.
Creative Biolabs: HEYL Antibodies for Research
Creative Biolabs specializes in the production of high-quality HEYL antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom HEYL Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our HEYL antibodies, custom preparations, or technical support, contact us at email.
Reference
- Sun, Hao, et al. "Silencing of NOTCH3 signaling in meniscus smooth muscle cells inhibits fibrosis and exacerbates degeneration in a HEYL‐dependent manner." Advanced Science 10.16 (2023): 2207020. https://doi.org/10.1002/advs.202207020
Anti-HEYL antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ATP1B3 Recombinant Antibody (1E9) (CBMAB-A4021-YC)

-
Mouse Anti-FYN Recombinant Antibody (10) (CBMAB-S6332-CQ)

-
Mouse Anti-BANF1 Recombinant Antibody (3F10-4G12) (CBMAB-A0707-LY)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-0790) (CBMAB-0793-YY)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Rabbit Anti-AP2M1 (Phosphorylated T156) Recombinant Antibody (D4F3) (PTM-CBMAB-0610LY)

-
Mouse Anti-BAD (Phospho-Ser136) Recombinant Antibody (CBYY-0138) (CBMAB-0139-YY)

-
Mouse Anti-AHCYL1 Recombinant Antibody (V2-180270) (CBMAB-A1703-YC)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Mouse Anti-APCS Recombinant Antibody (CBYC-A663) (CBMAB-A3054-YC)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-AMOT Recombinant Antibody (CBYC-A564) (CBMAB-A2552-YC)

-
Mouse Anti-GFAP Recombinant Antibody (24) (CBMAB-G2927-LY)

-
Mouse Anti-8-oxoguanine Recombinant Antibody (V2-7697) (CBMAB-1869CQ)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Mouse Anti-ESR1 Recombinant Antibody (Y31) (CBMAB-1208-YC)

-
Mouse Anti-AQP2 Recombinant Antibody (G-3) (CBMAB-A3359-YC)

-
Mouse Anti-EGR1 Recombinant Antibody (CBWJZ-100) (CBMAB-Z0289-WJ)

-
Mouse Anti-CRYAB Recombinant Antibody (A4345) (CBMAB-A4345-YC)

-
Mouse Anti-AZGP1 Recombinant Antibody (CBWJZ-007) (CBMAB-Z0012-WJ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






