MIOX Antibodies
Background
MIOX (inositol oxygenase) is a soluble enzyme protein existing within cells, mainly distributed in the kidney and liver tissues of mammals. This enzyme catalyzes the oxidative cleavage reaction of inositol, participates in the regulation of inositol metabolic pathways, and plays a significant role in maintaining intracellular osmotic pressure balance and signal transduction processes. Under metabolic stress conditions, MIOX participates in cellular stress response and energy metabolism regulation by influencing the generation of phosphoinositol derivatives. This gene was first cloned and identified in 1996. The protein it encodes adopts a β -propeller three-dimensional structure, and the active center contains a conserved iron ion binding site. The research on its structure and function has deepened people's understanding of the association between the glucose metabolism network and metabolic diseases, providing a molecular basis for the study of related pathological mechanisms.
Structure of MIOX
MIOX is an enzyme protein with a relatively small molecular weight, approximately 32 kDa. Its specific molecular weight varies slightly among different species, mainly due to minor variations in amino acid sequences.
| Species | Human | Mouse | Rat |
| Molecular Weight (kDa) | 32.0 | 31.8 | 31.9 |
| Primary Structural Differences | Conservative catalytic domain | High homology and high sequence similarity | Active center exactly the key amino acids |
The MIOX protein is composed of approximately 298 amino acids and folds to form a typical (β/α) 8-barrel-shaped tertiary structure. The core domain of this protein contains a diiron binding site composed of conserved histidine and aspartic acid residues, which is the key active center for its catalytic inositol oxidative cleavage reaction. The activity of the enzyme depends on the binding of divalent iron ions, and its overall structure forms a hydrophobic channel to facilitate the entry and localization of the substrate inositol.
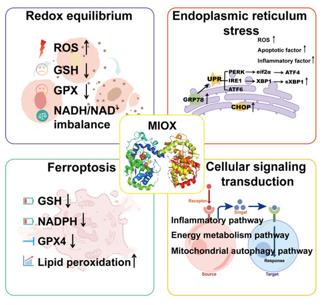 Fig. 1 The function of MIOX.1
Fig. 1 The function of MIOX.1
Key structural properties of MIOX:
- Typical (β/α) 8-barrel folding structure
- By conservative histidine and aspartic acid residue consisting of double iron active center
- Iron-dependent catalytic sites are involved in the oxidative cleavage of inositol
Functions of MIOX
The main function of MIOX is to catalyze inositol metabolism and regulate osmotic pressure balance. In addition, it is also involved in a series of physiological and pathological processes, including oxidative stress response and energy metabolism regulation.
| Function | Description |
| Inositol metabolism | Catalyzing the oxidative cleavage of inositol to D-glucuronic acid is the rate-limiting step in the catabolism of inositol. |
| Osmotic pressure regulation | By influencing the concentration of inositol in the cell and its phosphorylation derivatives, involved in maintenance of osmotic pressure steady. |
| Energy metabolism | Its catalytic product into the sugar metabolic pathways, precursor for cell energy supply. |
| Stress response | Under the condition of oxidative stress or high sugar expression, participate in cell adaptive response. |
| Associated with diseases | The abnormal expression associated with diabetic nephropathy, metabolic syndrome and other pathological process. |
The catalytic efficiency of MIOX is influenced by the substrate concentration and the intracellular REDOX state. Its activity is closely related to metabolic requirements and plays a key metabolic node role in the kidneys and liver.
Applications of MIOX and MIOX Antibody in Literature
- Wang, Yiqiu, et al. "Integrated analysis of MIOX gene in prognosis of clear-cell renal cell carcinoma." Cell Death & Disease 16.1 (2025): 368.https://doi.org/10.1038/s41419-025-07698-7
Based on TCGA data, this study, through single-cell and spatial transcriptome analysis, for the first time clearly identified the specific expression and independent prognostic value of MIOX in clear cell renal cell carcinoma, suggesting that it can serve as a potential therapeutic target.
- Zhang, Ying, et al. "Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA." Cell Death & Differentiation 29.9 (2022): 1850-1863. https://doi.org/10.1038/s41418-022-00970-9
Studies have found that NEAT1 upregulates MIOX expression by competitively binding to miR-362-3p, thereby enhancing ROS accumulation and NADPH/GSH depletion caused by ferroptosis inducers, and ultimately promoting tumor ferroptosis.
- Han, Shaocong, et al. "Myo-Inositol Oxygenase (MIOX): A Pivotal Regulator and Therapeutic Target in Multiple Diseases." Current Issues in Molecular Biology 47.9 (2025): 745. https://doi.org/10.3390/cimb47090745
Research has found that MIOX is a key enzyme in inositol metabolism in mammals. Its dysfunction can disrupt metabolic balance, trigger oxidative stress, inflammation and ferroptosis, and subsequently lead to various diseases such as tumors. In the future, it is necessary to deeply explore its pathogenic mechanism and develop targeted regulators.
- Zhou, Wenjun, Congyi Yu, and Yiwen Long. "Myo‐inositol oxygenase (MIOX) accelerated inflammation in the model of infection‐induced cardiac dysfunction by NLRP3 inflammasome." Immunity, Inflammation and Disease 11.5 (2023): e829. https://doi.org/10.1002/iid3.829
Studies have confirmed that in infectious myocardial dysfunction, MIOX enhances the activity of NLRP3 inflammasomes by inhibiting the degradation of NLRP3 proteins, thereby intensifying the inflammatory response. Inhibiting MIOX can effectively alleviate this pathological process.
- Sharma, Isha, Fei Deng, and Yashpal S. Kanwar. "Modulation of renal injury by variable expression of Myo-Inositol Oxygenase (MIOX) via perturbation in metabolic sensors." Biomedicines 8.7 (2020): 217. https://doi.org/10.3390/biomedicines8070217
Research has revealed that in obesity-related kidney injury, the renal tubular enzyme MIOX aggravates the imbalance of cellular energy homeostasis and mitochondrial dysfunction by inhibiting metabolic sensors such as AMPK and Sirt1, thereby promoting the development of chronic kidney disease.
Creative Biolabs: MIOX Antibodies for Research
Creative Biolabs specializes in the production of high-quality MIOX antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MIOX Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MIOX antibodies, custom preparations, or technical support, contact us at email.
Reference
- Han, Shaocong, et al. "Myo-Inositol Oxygenase (MIOX): A Pivotal Regulator and Therapeutic Target in Multiple Diseases." Current Issues in Molecular Biology 47.9 (2025): 745. https://doi.org/10.3390/cimb47090745
Anti-MIOX antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Mouse Anti-dsRNA Recombinant Antibody (2) (CBMAB-D1807-YC)

-
Mouse Anti-BIRC7 Recombinant Antibody (88C570) (CBMAB-L0261-YJ)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-0790) (CBMAB-0793-YY)

-
Mouse Anti-ACTN4 Recombinant Antibody (V2-6075) (CBMAB-0020CQ)

-
Mouse Anti-APC Recombinant Antibody (CBYC-A661) (CBMAB-A3036-YC)

-
Rat Anti-ADGRE4 Recombinant Antibody (V2-160163) (CBMAB-F0011-CQ)

-
Mouse Anti-CAPZB Recombinant Antibody (CBYY-C0944) (CBMAB-C2381-YY)

-
Mouse Anti-AKT1 Recombinant Antibody (V2-180546) (CBMAB-A2070-YC)

-
Mouse Anti-FAS2 Monoclonal Antibody (1D4) (CBMAB-0071-CN)

-
Rabbit Anti-CCN1 Recombinant Antibody (CBWJC-3580) (CBMAB-C4816WJ)

-
Rat Anti-ADAM10 Recombinant Antibody (V2-179741) (CBMAB-A1103-YC)

-
Mouse Anti-ALPL Antibody (B4-78) (CBMAB-1009CQ)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Rabbit Anti-BAD (Phospho-Ser136) Recombinant Antibody (CAP219) (CBMAB-AP536LY)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Rat Anti-CD300A Recombinant Antibody (172224) (CBMAB-C0423-LY)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

-
Mouse Anti-ATP5F1A Recombinant Antibody (51) (CBMAB-A4043-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








