PHIP Antibodies
Background
The PHIP gene encodes a multi-domain scaffold protein, which is mainly present in the nucleus and cytoplasm. This protein participates in a variety of key biological processes such as DNA damage repair, cell cycle progression, and insulin signaling pathways by regulating histone acetylation modification and transcription factor activity. Research has found that PHIP gene mutations are closely related to neurodevelopmental abnormalities and the risk of diabetes, and its molecular mechanism involves the precise coordination of chromatin remodeling and signal transduction networks. Since its first identification in 2001, this gene has become a research hotspot due to its dual role in epigenetic regulation and metabolic diseases, providing an important model for revealing the function of protein-protein interaction networks in human diseases.
Structure of PHIP
PHIP is a large multi-domain scaffold protein with a molecular weight of approximately 320 kDa. This protein has highly conserved structural characteristics in different species, and its molecular weight may vary slightly due to transcript variations or post-translational modifications.
| Species | Human | Mouse | Zebrafish | Fruit fly |
| Molecular Weight (kDa) | 320 | 318 | 305 | 290 |
| Primary Structural Differences | Contains multiple PH domains and nuclear localization signals | The homology with human was 95% | Has duplicate WD40 domains | Simplified single PH domain |
The PHIP protein is composed of 2,862 amino acids, and its tertiary structure forms a flexible scaffold conformation through a series of PH domains, WD40 repeat sequences, and nuclear output signal domains. This protein specifically binds to histone acetylation modification through the bromine domain at the C-terminal, while the PH domain at the N-terminal mediates membrane-lipid interactions and signal transduction. This multi-domain assembly method enables it to synergistically regulate the assembly of transcriptional complexes and membrane receptor signal transduction.
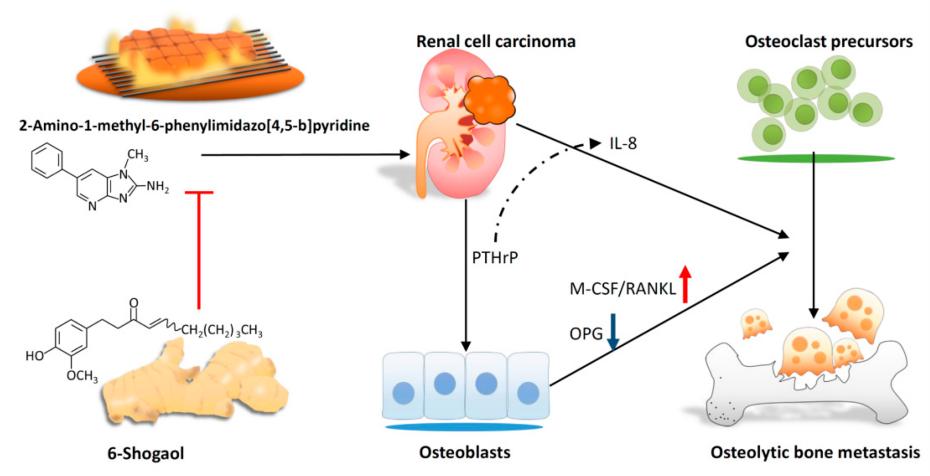 Fig. 1 Scheme of proposed 6-shogaol-inhibited PhIP-induced human renal cell carcinoma bone metastasis.1
Fig. 1 Scheme of proposed 6-shogaol-inhibited PhIP-induced human renal cell carcinoma bone metastasis.1
Key structural properties of PHIP:
- Assembly of multiple functional domains
- Hydrophobic regions mediate protein interactions and complex assembly
- Bromine domains specifically recognize histone acetylation modifications
Functions of PHIP
The main function of PHIP protein is to act as a molecular scaffold to participate in transcriptional regulation and signal transduction. In addition, it also involves a variety of cellular processes such as chromatin remodeling, cell cycle regulation and maintenance of metabolic homeostasis.
| Function | Description |
| Transcriptional co-activation | Recognize acetylated histones through bromine domains, recruit transcriptional complexes to specific gene promoter regions, and regulate gene expression. |
| Insulin signaling regulation | As a scaffold protein, it integrates IRS1 with downstream effector molecules to enhance the conduction efficiency of the insulin signaling pathway. |
| DNA damage response | Participate in double-stranded DNA fracture repair process, by adjusting the histone modification and repair protein stability maintaining genome. |
| Cell cycle process | By regulating the expression of cell cycle-related genes such as Cyclin D1, it affects the G1/S phase transition. |
| Maintenance of metabolic homeostasis | Regulate glucose uptake in the liver and adipose tissue and lipid metabolism related gene transcription, affect the whole body metabolic balance. |
PHIP forms a dynamic interaction network through its multi-domain assembly, responding to intracellular and extracellular signals in a synergistic manner. Its dysfunction is closely related to cancer, diabetes and neurodevelopmental disorders.
Applications of PHIP and PHIP Antibody in Literature
1. Malik, Durr-e-shahwar, Rhiannon M. David, and Nigel J. Gooderham. "Ethanol potentiates the genotoxicity of the food-derived mammary carcinogen PhIP in human estrogen receptor-positive mammary cells: Mechanistic support for lifestyle factors (cooked red meat and ethanol) associated with mammary cancer." Archives of Toxicology 92.4 (2018): 1639-1655. https://doi.org/10.1007/s00204-018-2160-9
The article indicates that both PhIP and ethanol produced by consuming processed meat can induce cytotoxicity in breast cells. PhIP enhances DNA damage by activating the CYP1B1 and ER-α pathways, while ethanol synergistically intensifies the genotoxic effect of PhIP through oxidative stress, especially in ER-α -positive cells, thereby increasing the risk of breast cancer.
2. Zapico Linares, Aida, et al. "Potential of Fiber and Probiotics to Fight Against the Effects of PhIP+ DSS-Induced Carcinogenic Process of the Large Intestine." American Chemical Society 72(45): 25161–25172 (2024). https://doi.org/10.1021/acs.jafc.4c07366
Studies have shown that dietary fiber and probiotics can alleviate colonic mucosal injury in rats induced by the combination of PhIP and DSS. Fiber supplementation reduces crypt loss and inflammation, while probiotics increase colon length. Both regulate the intestinal flora and reduce the abundance of pro-inflammatory bacteria, thereby countering the intestinal toxicity of PhIP.
3. Griggs, Amy M., et al. "2-Amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) is selectively toxic to primary dopaminergic neurons in vitro." Toxicological Sciences 140.1 (2014): 179-189. https://doi.org/10.1093/toxsci/kfu060
Studies have found that PhIP and its metabolite N-OH-PhIP produced by dietary processing have selective toxicity to dopaminergic neurons, which can lead to neuronal reduction and oxidative damage. However, blueberry extract can effectively alleviate its neurotoxic effects through antioxidant mechanisms.
4. Akhtar, Shabana, et al. "Ex vivo/in vitro protective effect of myricetin bulk and nano-forms on PhIP-induced DNA damage in lymphocytes from healthy individuals and pre-cancerous MGUS patients." Archives of Toxicology 94.7 (2020): 2349-2357. https://doi.org/10.1007/s00204-020-02754-x
Research has found that the dietary mutagen PhIP can induce DNA damage in lymphocytes. The flavonoid myxidine, especially its nanoform, can significantly inhibit DNA strand breaks and micronucleus formation caused by PhIP, and has a better protective effect on lymphocytes in patients with precancerous lesions. The mechanism may be related to the regulation of gene pathways such as P53.
5. Yeh, I-Jeng, et al. "6-Shogaol suppresses 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP)-induced human 786-O renal cell carcinoma osteoclastogenic activity and metastatic potential." Nutrients 11.10 (2019): 2306. https://doi.org/10.3390/nu11102306
Research has found that the dietary carcinogen PhIP can promote the secretion of PTHrP by renal cancer cells, thereby regulating the bone microenvironment, enhancing the activity of osteoclasts, and leading to bone metastasis. The active component 6-gingerol of ginger can effectively inhibit PHIP-mediated bone resorption.
Creative Biolabs: PHIP Antibodies for Research
Creative Biolabs specializes in the production of high-quality PHIP antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom Myoglobin Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our PHIP antibodies, custom preparations, or technical support, contact us at email.
Reference
- Yeh, I-Jeng, et al. "6-Shogaol suppresses 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP)-induced human 786-O renal cell carcinoma osteoclastogenic activity and metastatic potential." Nutrients 11.10 (2019): 2306. https://doi.org/10.3390/nu11102306
Anti-PHIP antibodies
 Loading...
Loading...
Hot products 
-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-ALB Recombinant Antibody (V2-363290) (CBMAB-S0173-CQ)

-
Mouse Anti-ENO1 Recombinant Antibody (8G8) (CBMAB-E1329-FY)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

-
Mouse Anti-BAD (Phospho-Ser136) Recombinant Antibody (CBYY-0138) (CBMAB-0139-YY)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Mouse Anti-C4B Recombinant Antibody (CBYY-C2996) (CBMAB-C4439-YY)

-
Mouse Anti-CFL1 (Phospho-Ser3) Recombinant Antibody (CBFYC-1770) (CBMAB-C1832-FY)

-
Mouse Anti-HTLV-1 gp46 Recombinant Antibody (CBMW-H1006) (CBMAB-V208-1154-FY)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Rat Anti-FABP3 Recombinant Antibody (CBXF-2299) (CBMAB-F1612-CQ)

-
Mouse Anti-EPO Recombinant Antibody (CBFYR0196) (CBMAB-R0196-FY)

-
Mouse Anti-AGK Recombinant Antibody (V2-258056) (CBMAB-M0989-FY)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-CCDC6 Recombinant Antibody (CBXC-0106) (CBMAB-C5397-CQ)

-
Mouse Anti-AOC3 Recombinant Antibody (CBYY-0014) (CBMAB-0014-YY)

-
Mouse Anti-NSUN6 Recombinant Antibody (D-5) (CBMAB-N3674-WJ)

-
Mouse Anti-CD83 Recombinant Antibody (HB15) (CBMAB-C1765-CQ)

-
Mouse Anti-ENO2 Recombinant Antibody (H14) (CBMAB-E1341-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








