SCO2 Antibodies
Background
The SCO2 gene is located on human chromosome 22q13.2 and encodes a key assembly protein for the synthesis of cytochrome c oxidase (COX), which is the core catalytic subunit of mitochondrial respiratory chain complex IV. Its encoded products directly participate in maintaining the balance between cellular energy metabolism and aerobic respiration by regulating the biogenesis process of cytochrome c oxidase. Mutations in this gene can lead to tissue-specific COX deficiency, causing early-onset fatal cardiomyopathy and neuromuscular disorders. In 2000, Papadopoulou and other researchers first reported its correlation with human diseases. Its pathogenic mechanism is closely related to mitochondrial oxidative phosphorylation dysfunction and has become an important molecular target for studying energy metabolism diseases and mitochondrial quality control mechanisms.
Structure of SCO2
Myoglobin is a relatively small protein with a molecular weight of approximately 16.7 kDa. This weight may slightly vary between species due to minor differences in amino acid sequence.
| Species | Human | Mouse | Bovine | Zebrafish | Yeast |
| Molecular Weight (kDa) | 28.3 | 28.1 | 28.2 | 27.8 | 26.5 |
| Primary Structural Differences | Contains the conserved cytochrome c oxidase binding domain | With the human sequence homology is as high as 85% | Core functional areas are highly conservative | With typical SCO vertebrates structure domain | Although it has evolved a long time ago, it retains the key coordination sites of copper ions |
The tertiary structure of this protein forms a conserved metal-binding core, in which two key cysteine residues (Cys-136 and Cys-158 in human SCO2) coordinate with a copper ion, constituting the structural basis of its copper chaperone activity. Its C-terminal domain is responsible for the interaction with the mitochondrial membrane and the cytochrome C oxidase subunit, while the N-terminal transmembrane region ensures its correct localization on the membrane. This precise conformation enables SCO2 to specifically transport copper ions to the catalytic center of cytochrome c oxidase, which is crucial for the maturation of respiratory chain complex IV.
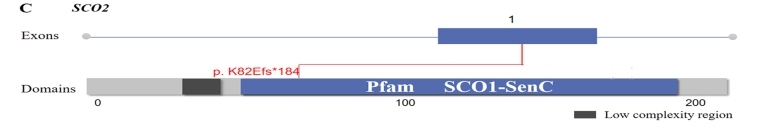 Fig. 1 Location of identified variants in the SCO2.1
Fig. 1 Location of identified variants in the SCO2.1
Key structural properties of SCO2:
- Conserved SCO domains constitute the protein core
- Across the membrane anchoring area coordination center synergy with copper ions
- Cysteine residues (Cys136/158) form copper binding sites
Functions of SCO2
The core function of the SCO2 gene is to participate in the biosynthesis and assembly of cytochrome c oxidase (COX). Its main physiological roles are as follows:
| Function | Description |
| Copper ion transport | As a specific copper molecular chaperone, it precisely transports copper ions to the CuA site of the COX catalytic subunit. |
| Regulation of oxidative phosphorylation | By ensuring the maturation of complex IV, it directly maintains the function of the mitochondrial respiratory chain and the efficiency of ATP production. |
| Cellular energy metabolism balance | Coordination of tissue oxygen demand and respiratory chain activity plays a key role in energy-intensive tissues such as myocardium and nerves. |
| Mitochondrial quality control | Functional defects activate mitochondrial autophagy and affect the homeostasis regulation of reactive oxygen species (ROS). |
| Embryo development support | Pathogenic mutations are indispensable for the normal development of the heart and nervous system and can lead to an early fatal phenotype. |
This protein achieves precise metal ion delivery through its conserved copper coordination center, and its functional efficiency directly determines the catalytic activity of cytochrome c oxidase, thereby influencing the overall cellular energy metabolism level.
Applications of SCO2 and SCO2 Antibody in Literature
1. Miliotou, Androulla N., et al. "Protein transduction domain-mediated delivery of recombinant proteins and in vitro transcribed mRNAs for protein replacement therapy of human severe genetic mitochondrial disorders: the case of SCO2 deficiency." Pharmaceutics 15.1 (2023): 286. https://doi.org/10.3390/pharmaceutics15010286
The article indicates that mutations in the SCO2 gene lead to mitochondrial CoX-deficient cardiomyopathy. The study successfully restored the COX activity of fibroblasts in patients by using TAT-mediated Sco2 protein and mRNA replacement therapy, demonstrating the potential for clinical transformation.
2. Hallas, Tova, et al. "Investigating the cardiac pathology of SCO2‐mediated hypertrophic cardiomyopathy using patients induced pluripotent stem cell–derived cardiomyocytes." Journal of cellular and molecular medicine 22.2 (2018): 913-925. https://doi.org/10.1111/jcmm.13392
In this study, cardiomyocytes differentiated from IPscs of patients with SCO2 mutations were utilized to reveal for the first time the pathological mechanism of arrhythmia caused by this disease. Cells exhibit abnormal mitochondrial structure, weakened response to positive inotropic drugs, and impaired calcium processing function due to insufficient ATP, leading to delayed depolarization and increased heart rate variability.
3. Zheng, Yi-Han, et al. "Mutational screening of AGRN, SLC39A5, SCO2, P4HA2, BSG, ZNF644, and CPSF1 in a Chinese cohort of 103 patients with nonsyndromic high myopia." Molecular Vision 27 (2021): 706. http://www.molvis.org/molvis/v27/706
In this study, cardiomyocytes differentiated from IPscs of patients with SCO2 mutations were utilized to reveal for the first time the pathological mechanism of arrhythmia caused by this disease. Cells exhibit abnormal mitochondrial structure, weakened response to positive inotropic drugs, and impaired calcium processing function due to insufficient ATP, leading to delayed depolarization and increased heart rate variability.
4. Chadha, Radhika, Ritika Shah, and Shalini Mani. "Analysis of reported SCO2 gene mutations affecting cytochrome c oxidase activity in various diseases." Bioinformation 10.6 (2014): 329. https://doi.org/10.6026/97320630010329
The article indicates that there are numerous types of SCO2 gene mutations, but the effects of most of them on protein functions are unknown. This study analyzed multiple SCO2 non-synonym mutations through bioinformatics tools, predicting that they would damage protein structure and potentially lead to cytochrome C oxidase functional defects, providing a basis for subsequent experimental verification and disease diagnosis.
5. Barcia, Giulia, et al. "Cytochrome c oxidase deficiency caused by biallelic SCO2 mutations in two sibs with cerebellar ataxia and progressive peripheral axonal neuropathy." Molecular Genetics and Metabolism Reports 21 (2019): 100528. https://doi.org/10.1016/j.ymgmr.2019.100528
This study reports a novel homozygous SCO2 gene mutation. The patient presented with cerebellar ataxia and progressive peripheral neuropathy and survived for a long time. This discovery expands the clinical spectrum related to SCO2 mutations, suggesting that it can lead to atypical cases with relatively mild symptoms and no cardiomyopathy.
Creative Biolabs: SCO2 Antibodies for Research
Creative Biolabs specializes in the production of high-quality SCO2 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom SCO2 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our SCO2 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Zheng, Yi-Han, et al. "Mutational screening of AGRN, SLC39A5, SCO2, P4HA2, BSG, ZNF644, and CPSF1 in a Chinese cohort of 103 patients with nonsyndromic high myopia." Molecular Vision 27 (2021): 706. http://www.molvis.org/molvis/v27/706
Anti-SCO2 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-BACE1 Recombinant Antibody (CBLNB-121) (CBMAB-1180-CN)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-CSPG4 Recombinant Antibody (CBFYM-1050) (CBMAB-M1203-FY)

-
Mouse Anti-FN1 Monoclonal Antibody (D6) (CBMAB-1240CQ)

-
Mouse Anti-BIRC3 Recombinant Antibody (16E63) (CBMAB-C3367-LY)

-
Mouse Anti-2C TCR Recombinant Antibody (V2-1556) (CBMAB-0951-LY)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Rabbit Anti-BRCA2 Recombinant Antibody (D9S6V) (CBMAB-CP0017-LY)

-
Rabbit Anti-ENO2 Recombinant Antibody (BA0013) (CBMAB-0272CQ)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-ATP1B3 Recombinant Antibody (1E9) (CBMAB-A4021-YC)

-
Mouse Anti-8-oxoguanine Recombinant Antibody (V2-7719) (CBMAB-1898CQ)

-
Mouse Anti-FOSB Recombinant Antibody (CBXF-3593) (CBMAB-F2522-CQ)

-
Mouse Anti-CORO1A Recombinant Antibody (4G10) (V2LY-1206-LY806)

-
Mouse Anti-ASH1L Monoclonal Antibody (ASH5H03) (CBMAB-1372-YC)

-
Mouse Anti-AQP2 Recombinant Antibody (E-2) (CBMAB-A3358-YC)

-
Mouse Anti-CD33 Recombinant Antibody (6C5/2) (CBMAB-C8126-LY)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-CRTAM Recombinant Antibody (CBFYC-2235) (CBMAB-C2305-FY)

-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








