TLR9 Antibodies
Background
TLR9 is a pattern recognition receptor located in the inner membrane of cells, mainly distributed in the endosomal compartments of immune cells such as B cells and dendritic cells. The protein encoded by this gene can specifically recognize the unmethylated CpG DNA sequence, a structure commonly found in the genomes of bacteria and viruses, thereby activating the innate immune response and inducing the secretion of inflammatory factors. In 1994, the Akira team first discovered the immune function mechanism of TLR9 in mice. The research on this mechanism has provided a new target for the development of vaccine adjuvants and the treatment of autoimmune diseases. Its unique nucleic acid perception function reveals the molecular basis by which vertebrates distinguish "self" from "non-self" through Toll-like receptor families. The related research won the Nobel Prize in Physiology or Medicine in 2011. The research on the signal transduction pathway of TLR9 has greatly promoted the development of fields such as infection immunity and tumor immunotherapy.
Structure of TLR9
TLR9 is a transmembrane protein with a molecular weight of approximately 115-120 kDa. Its precise molecular weight varies slightly among different species, mainly due to the length polymorphism of extracellular leucine-rich repeat (LRR) domains.
| Species | Human | Mouse | Rhesus monkey | Rat |
| Molecular Weight (kDa) | 116.2 | 115.8 | 117.1 | 115.5 |
| Primary Structural Differences | Contains 26 LRRS | 25 LRRS | 27 LRRS | 24 LRRS |
This protein is composed of an N-terminal signal peptide (1-24aa), an extracellular LRR recognition domain (25-650aa), a transmembrane region (651-672aa), and an intracellular TIR signal domain (673-1032aa). Its tertiary structure forms a horseshoe conformation through the LRR domain, specifically recognizing the phosphodiester skeleton of unmethylated CpG DNA.
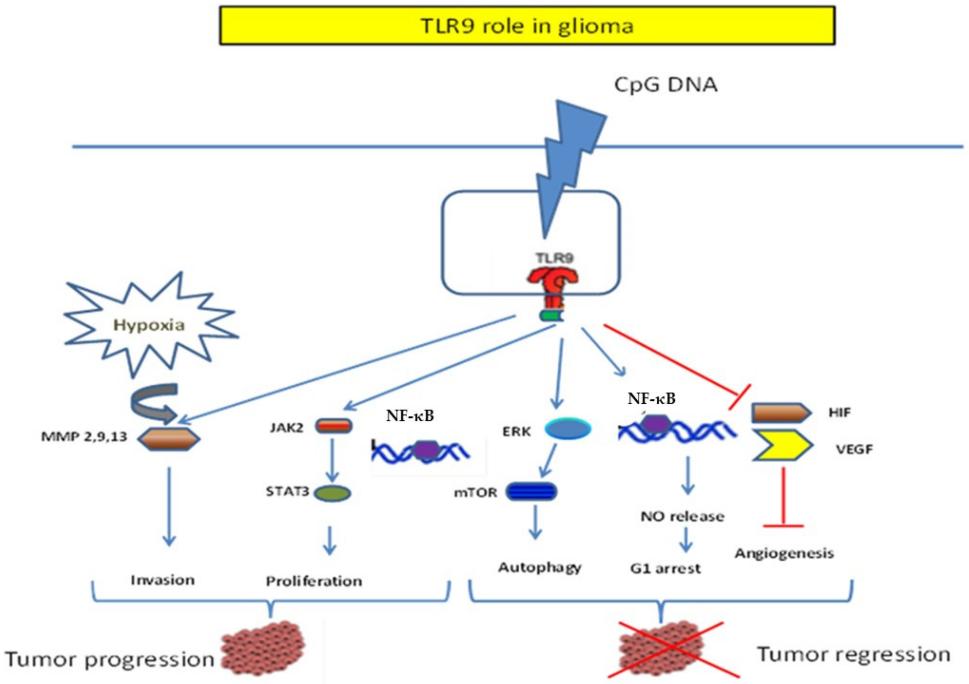 Fig. 1 Dichotomic role of TLR9 in Glioma.1
Fig. 1 Dichotomic role of TLR9 in Glioma.1
Key structural properties of TLR9:
- Helical-β lamellar structures rich in leucine repeat sequences (LRR)
- Hydrophobic binding pocket with positive charge
- Zinc finger-like metal ion binding sites
Functions of TLR9
TLR9 is a key pattern recognition receptor in the innate immune system, mainly recognizing the DNA characteristics of pathogens and simultaneously participating in the regulation of autoimmune balance. Its functional diversity is reflected in the following aspects:
| Function | Description |
| Pathogen DNA identification | Specifically binding to the unmethylated CpG motif in bacterial/viral DNA, it triggers the release of inflammatory factors through the MyD88 pathway. |
| Autoimmune regulation | Endosome localization characteristics avoid recognizing nuclear DNA, and PH-dependent activation can prevent abnormal reactions to host DNA. |
| Vaccine adjuvant effect | CpG-ODN (oligodeoxynucleotide), as a TLR9 ligand, enhances B-cell antibody production and Th1-type immune response. |
| Anti-tumor immunity | Activate dendritic cells to cross-present tumor antigens and promote the activation of CD8+ T cells (dependent on IRF7 signaling branches). |
| Intestinal flora homeostasis maintenance | By identifying the DNA of symbiotic bacteria to regulate the intestinal epithelial barrier function, it is prone to cause inflammatory bowel disease when deficient. |
The signal dynamics of TLR9 exhibits biphasic activation characteristics:
- Early response (15-30 minutes): The endosomal compartment triggers IRAK4/ TrAF6-dependent rapid activation of NF-κB.
- Continuous response (4-6 hours): Endosome-lysosomal transport induces phosphorylation of IRF7 to produce type I interferon.
Applications of TLR9 and TLR9 Antibody in Literature
1. Fehri, Emna, et al. "TLR9 and glioma: Friends or foes?." Cells 12.1 (2022): 152. https://doi.org/10.3390/cells12010152
The article indicates that TLR9 has a dual role in glioma: On the one hand, it promotes tumor invasion and hypoxia by activating matrix metalloproteinases. On the other hand, its agonist CpG ODN can enhance radiosensitivity, promote the anti-tumor effect of T cells and the presentation ability of microglia antigens, while inhibiting Treg cells. It is expected to become a new target for immunotherapy, but its application should be cautious.
2. Ni, Hai, et al. "Cyclical palmitoylation regulates TLR9 signalling and systemic autoimmunity in mice." Nature Communications 15.1 (2024): 1. https://doi.org/10.1038/s41467-023-43650-z
The article indicates that the S-palmitoylation modification of TLR9 regulates the autoimmune response: DHHC3 palmitoylates TLR9 in the Golgi body to promote its transport, while PPT1 depalmitoylates TLR9 in the lysosome, causing it to detse from UNC93B1 and enhancing the secretion of IFNα and TNF by pDC and macrophages. Inhibiting PPT1 can alleviate nephritis in SLE model mice and reduce the production of IFNα in patients.
3. Shepard, Christopher R. "TLR9 in MAFLD and NASH: At the Intersection of Inflammation and Metabolism." Frontiers in endocrinology 11 (2021): 613639. https://doi.org/10.3389/fendo.2020.613639
The article indicates that excessive activation of TLR9 plays a key role in obesity and NASH: chronic overnutrition leads to the release of mitochondrial DNA (mtDNA), which continuously activates TLR9 and drives liver inflammation and fibrosis. Inhibiting TLR9 may become a potential therapeutic strategy that targets both metabolic disorders and inflammation simultaneously.
4. Zhou, Binghai, et al. "Hepatoma cell-intrinsic TLR9 activation induces immune escape through PD-L1 upregulation in hepatocellular carcinoma." Theranostics 10.14 (2020): 6530. https://doi.org/10.7150/thno.44417
The article indicates that the combination of TLR9 agonists and PD-1 inhibitors promotes PD-L1 expression by regulating PARP1 self-modification and STAT3 phosphorylation, thereby enhancing immune escape in liver cancer. Mechanically, TLR9 increases PARP1 ubiquitination degradation by inhibiting PARG, reduces STAT3 PARylation and enhances its phosphorylation, and ultimately boosts PD-L1 transcription. This combined therapy significantly inhibits the growth of liver cancer.
5. Suthers, Amy N., and Stefanie Sarantopoulos. "TLR7/TLR9-and B cell receptor-signaling crosstalk: promotion of potentially dangerous B cells." Frontiers in immunology 8 (2017): 775. https://doi.org/10.3389/fimmu.2017.00775
The article indicates that TLR7/9 regulates the autoreactivity of B cells by coordinating with BCR signaling: TLR9 not only participates in the normal response of memory B cells but also promotes the production of autoantibodies, while inhibiting TLR7-mediated autoimmunity. In high-nucleic acid environments such as allogeneic hematopoietic stem cell transplantation, the TLR-BCR synergistic signaling may exacerbate diseases such as chronic GVHD, and in-depth research on its molecular mechanism is needed to develop targeted therapies.
Creative Biolabs: TLR9 Antibodies for Research
Creative Biolabs specializes in the production of high-quality TLR9 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom TLR9 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our TLR9 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Fehri, Emna, et al. "TLR9 and glioma: Friends or foes?." Cells 12.1 (2022): 152. https://doi.org/10.3390/cells12010152
Anti-TLR9 antibodies
 Loading...
Loading...
Hot products 
-
Rabbit Anti-ALOX5AP Recombinant Antibody (CBXF-1219) (CBMAB-F0750-CQ)

-
Mouse Anti-CECR2 Recombinant Antibody (CBWJC-2465) (CBMAB-C3533WJ)

-
Mouse Anti-ALPL Antibody (B4-78) (CBMAB-1009CQ)

-
Mouse Anti-CCS Recombinant Antibody (CBFYC-1093) (CBMAB-C1150-FY)

-
Mouse Anti-ARID1B Recombinant Antibody (KMN1) (CBMAB-A3546-YC)

-
Rat Anti-ADGRE4 Recombinant Antibody (V2-160163) (CBMAB-F0011-CQ)

-
Mouse Anti-ARG1 Recombinant Antibody (CBYCL-103) (CBMAB-L0004-YC)

-
Mouse Anti-ANXA7 Recombinant Antibody (A-1) (CBMAB-A2941-YC)

-
Mouse Anti-BACE1 Recombinant Antibody (61-3E7) (CBMAB-1183-CN)

-
Mouse Anti-CTCF Recombinant Antibody (CBFYC-2371) (CBMAB-C2443-FY)

-
Mouse Anti-CD83 Recombinant Antibody (HB15) (CBMAB-C1765-CQ)

-
Rabbit Anti-ALDOA Recombinant Antibody (D73H4) (CBMAB-A2314-YC)

-
Mouse Anti-APP Recombinant Antibody (DE2B4) (CBMAB-1122-CN)

-
Mouse Anti-ADRB2 Recombinant Antibody (V2-180026) (CBMAB-A1420-YC)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503416) (CBMAB-V208-1402-FY)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-1728) (CBMAB-2077-YY)

-
Mouse Anti-APOE Recombinant Antibody (A1) (CBMAB-0078CQ)

-
Mouse Anti-2C TCR Recombinant Antibody (V2-1556) (CBMAB-0951-LY)

-
Mouse Anti-ALX1 Recombinant Antibody (96k) (CBMAB-C0616-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








