TOP1 Antibodies
Background
TOP1 is a key helicase widely present in the nucleus of eukaryotes. It regulates superhelical tension by reversibly cutting and reconnecting single DNA strands, ensuring the normal operation of core life processes such as DNA replication, transcription, and chromosome separation. Cancer cells are highly dependent on the activity of this enzyme due to their rapid proliferation characteristics, which makes TOP1 an important target for anti-tumor drugs (such as camptothecin-based preparations). In 1971, James Wang first discovered the activity of this enzyme in Escherichia coli. In 1987, the team led by Chinese scientist Wang Zhuo successfully analyzed its crystal structure and revealed a unique "rotary gate" catalytic mechanism - the enzyme molecule forms a covalent intermediate with DNA phosphodiester bonds through tyrosine residues, completing the topoisomerization reaction. As a model enzyme in the field of nucleic acid manipulation, the TOP1 study not only clarified the molecular basis for the dynamic maintenance of DNA, but also provided a theoretical framework for the development of new anti-cancer drugs and the understanding of the mechanism for maintaining genomic stability.
Structure of TOP1
TOP1 is a ribozyme with a molecular weight of approximately 91 kDa, and its precise molecular weight varies slightly among different species:
| Species | Human | Mice | Bovine | Yeast |
| Molecular Weight (kDa) | 90.7 | 89.2 | 91.3 | 85.6 |
| Primary Structural Differences | Containing 765 amino acids, C end structure domain is longer than the other | Highly homologous to humans | Conservative catalytic core | Relatively simple structure |
The unique "revolving door" mechanism enables it to instantly cut single-stranded DNA and reconnect after releasing helical tension through the tyrosine-phosphodiester bond covalent intermediate. This enzyme exhibits optimal activity under pH7.4 conditions. Mg²⁺ can enhance its stability, while camptothecin compounds can specifically capture the enzyme-DNA complex.
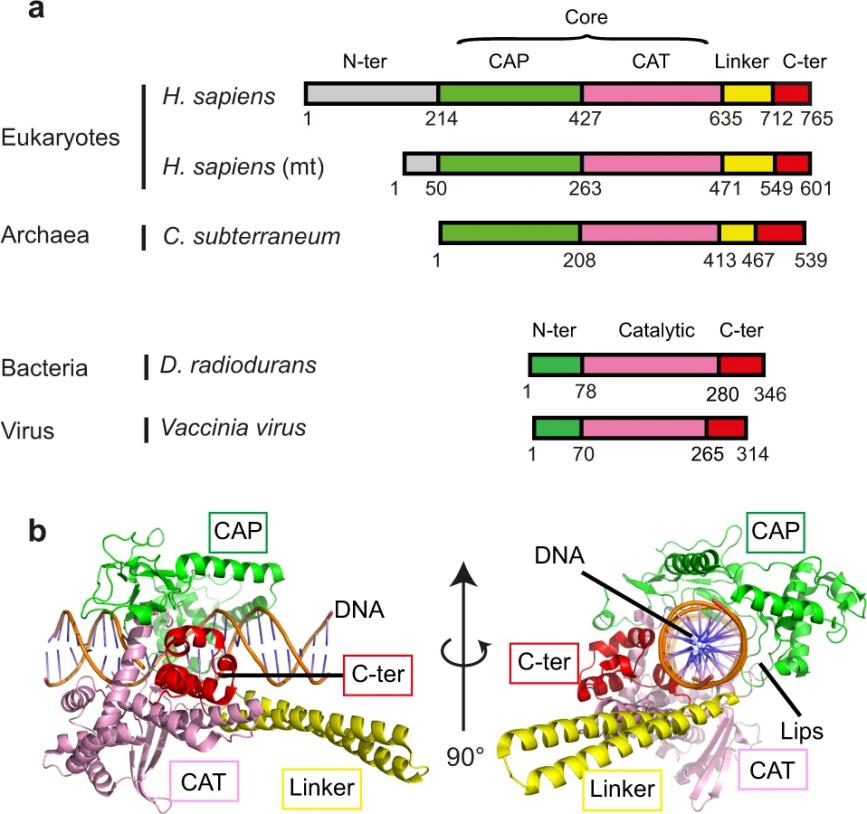 Fig. 1 Domains composition of type IB topoisomerases.1
Fig. 1 Domains composition of type IB topoisomerases.1
Key structural properties of TOP1:
- Characteristic "clip - core - connection zone" three-level structure
- Conservative catalytic core domain
- Unique "revolving door" mechanism
- Metal ion-dependent activity regulation
- Drug targeting sites of action
Functions of TOP1
The main function of TOP1 is to regulate the topological structure of DNA and play a key role in various cellular processes:
| Function | Description |
| DNA superhelix relaxation | By reversibly cutting and reconnecting DNA single strands, the superhelical tension generated during replication and transcription is eliminated. |
| Regulation of gene expression | Maintain the open state of chromatin and promote the binding of transcription factors to DNA. |
| Maintenance of genomic stability | Prevent DNA from breaking due to excessive entanglement and ensure accurate chromosome separation. |
| Cell cycle regulation | The activity significantly increased in the S phase and G2 phase, supporting DNA replication and damage repair. |
| Anti-cancer drug targets | Forms stable complexes with camptothecin compounds to induce apoptosis of cancer cells (e.g., irinotecan, topotecan). |
The catalytic efficiency of TOP1 shows a typical "ph-dependent" pattern, with the highest activity at a physiological pH of 7.4. Mg²⁺ can increase its activity by 3 to 5 times. Unlike type II topoisomerases, TOP1 only alters the number of DNA connections by ±1 each time it catalyzes. This precise regulation makes it a key molecule for maintaining genomic integrity.
Applications of TOP1 and TOP1 Antibody in Literature
1. Takahashi, Diane T., et al. "Topoisomerase I (TOP1) dynamics: conformational transition from open to closed states." Nature communications 13.1 (2022): 59.https://doi.org/10.1038/s41467-021-27686-7
The apo structure of archaeal thermally stable topoisomerase I (CsTOP1) reveals that it mediates the rotation of CAP and CAT modules through hinge regions, forming an open conformation to allow DNA entry and exit. Conserved tyrosine regulates conformational transformation during DNA binding, and hinge flexibility affects the sensitivity of the anti-cancer drug camptothecin.
2. Xu, Yang, and Chengtao Her. "Inhibition of topoisomerase (DNA) I (TOP1): DNA damage repair and anticancer therapy." Biomolecules 5.3 (2015): 1652-1670. https://doi.org/10.3390/biom5031652
Research has found that topoisomerase I (TOP1) inhibitors selectively kill rapidly proliferating cancer cells by hindering DNA replication. This article explores the characteristics of TOP1, the mechanism of action of its inhibitors, related DNA repair pathways, and its application in cancer treatment.
3. Marini, Victoria, et al. "MUS81 cleaves TOP1-derived lesions and other DNA–protein cross-links." BMC biology 21.1 (2023): 110. https://doi.org/10.1186/s12915-023-01614-1
Research has found that structure-specific endonuclease MUS81 can clet various protein-DNA cross-links (DPCs), but it can only function after the TOP1 part is degraded. MUS81 and TDP1 independently repair the damage induced by TOP1 inhibitors, and their synergistic absence enhances the sensitivity of cells to camptothecin, providing a new target for the combination of TOP1 inhibitors in anti-cancer treatment.
4. Zhang, Huimin, et al. "TDP1-independent pathways in the process and repair of TOP1-induced DNA damage." Nature communications 13.1 (2022): 4240. https://doi.org/10.1038/s41467-022-31801-7
Research has found that the TOP1-DNA crossover (TOP1cc) induced by the anti-cancer drug camptosine (CPT) can be directly repaired by TDP1 or converted into double-strand breaks (DSBs) by MUS81 and then repaired through the homologous recombination (HR) pathway. Genome-wide CRISPR screening revealed that APEX1/2 and TDP1 have synthetic lethal effects, but APEX deficiency does not affect the formation of DSBs in TDP1-deficient cells, indicating that there are two independent repair pathways for TOP1cc.
5. Rubio-Contreras, Diana, and Fernando Gómez-Herreros. "TDP1 suppresses chromosomal translocations and cell death induced by abortive TOP1 activity during gene transcription." Nature Communications 14.1 (2023): 6940. https://doi.org/10.1038/s41467-023-42622-7
Research reveals that DNA breaks mediated by TOP1 can be repaired by TDP1 or transformed into double-strand breaks (DSBs), which in turn trigger genomic rearrangements through MRN complexes and fault-prone non-homologous end junctions. This discovery clarifies the crucial role of TDP1-dependent repair mechanisms in maintaining transcriptional and genomic stability.
Creative Biolabs: TOP1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality TOP1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom TOP1 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our TOP1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Takahashi, Diane T., et al. "Topoisomerase I (TOP1) dynamics: conformational transition from open to closed states." Nature communications 13.1 (2022): 59.https://doi.org/10.1038/s41467-021-27686-7
Anti-TOP1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-EGR1 Recombinant Antibody (CBWJZ-100) (CBMAB-Z0289-WJ)

-
Mouse Anti-DMD Recombinant Antibody (D1190) (CBMAB-D1190-YC)

-
Mouse Anti-ADIPOR2 Recombinant Antibody (V2-179983) (CBMAB-A1369-YC)

-
Mouse Anti-AHCYL1 Recombinant Antibody (V2-180270) (CBMAB-A1703-YC)

-
Human Anti-SARS-CoV-2 S1 Monoclonal Antibody (CBFYR-0120) (CBMAB-R0120-FY)

-
Mouse Anti-ALOX5 Recombinant Antibody (33) (CBMAB-1890CQ)

-
Mouse Anti-AFDN Recombinant Antibody (V2-58751) (CBMAB-L0408-YJ)

-
Mouse Anti-AQP2 Recombinant Antibody (G-3) (CBMAB-A3359-YC)

-
Rabbit Anti-ENO2 Recombinant Antibody (BA0013) (CBMAB-0272CQ)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Mouse Anti-C4B Recombinant Antibody (CBYY-C2996) (CBMAB-C4439-YY)

-
Mouse Anti-CCN2 Recombinant Antibody (CBFYC-2383) (CBMAB-C2456-FY)

-
Rabbit Anti-DLK1 Recombinant Antibody (9D8) (CBMAB-D1061-YC)

-
Rabbit Anti-AKT2 (Phosphorylated S474) Recombinant Antibody (V2-556130) (PTM-CBMAB-0605LY)

-
Mouse Anti-CD83 Recombinant Antibody (HB15) (CBMAB-C1765-CQ)

-
Mouse Anti-CCDC25 Recombinant Antibody (CBLC132-LY) (CBMAB-C9786-LY)

-
Mouse Anti-ACO2 Recombinant Antibody (V2-179329) (CBMAB-A0627-YC)

-
Mouse Anti-DDC Recombinant Antibody (8E8) (CBMAB-0992-YC)

-
Rat Anti-CD63 Recombinant Antibody (7G4.2E8) (CBMAB-C8725-LY)

-
Mouse Anti-CCNH Recombinant Antibody (CBFYC-1054) (CBMAB-C1111-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








