ACADS Antibodies
Background
ACADS is A mitochondrial matrix enzyme mainly responsible for catalyzing the first step of the β -oxidation of short-chain fatty acids. This enzyme participates in the energy metabolism process by catalyzing the conversion of butyryl-CoA to 2-enbutyryl-CoA, and its expression is particularly significant in liver and muscle tissues. In 1985, researchers first purified the ACADS protein, and the analysis of its crystal structure provided a molecular basis for understanding fatty acid metabolic disorders. Mutations in the ACADS gene can lead to short-chain acyl-coA dehydrogenase deficiency (SCADD), which is clinically characterized by metabolic acidosis and developmental delay. In recent years, the role of ACADS in tumor metabolic reprogramming has also attracted increasing attention and has become an important target in the research of metabolic diseases and cancer treatment.
Structure of ACADS
ACADS is a mitochondrial enzyme with a molecular weight of approximately 44 kDa, and its molecular weight varies slightly among different species. The following is a comparison of ACADS characteristics of the main species:
| Species | Human | Mice | Rats | Bovine | Pigs |
| Molecular Weight (kDa) | 44.2 | 43.8 | 44.0 | 44.1 | 43.9 |
| Primary Structural Differences | Conservative catalytic domain | Gly94Asp polymorphism | Highly conserved sequence | Stable thermodynamic properties | Highly homologous to humans |
ACADS is composed of 412 amino acids and has the typical tertiary structural characteristics of acyl-coA dehydrogenase. Its active center contains the key glutamic acid residues (Glu368) and arginine residues (Arg256), which jointly participate in substrate binding and catalytic processes. The surface of enzyme molecules is distributed with multiple conserved hydrophobic pockets, which are specifically designed for recognizing short-chain fatty acid substrates. It is worth noting that the Gly185 and Ser188 residues of ACADS constitute oxygen-sensitive sites, a characteristic that makes them important targets for metabolic regulation.
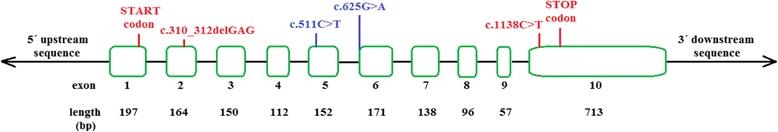 Fig. 1 The structure of the ACADS gene and the location of the frequent sequence variants.1
Fig. 1 The structure of the ACADS gene and the location of the frequent sequence variants.1
Key structural properties of ACADS:
- Conservative tetramer structure (α4 type)
- Hydrophobic active pockets recognize short-chain acyl-coenzyme A
- FAD excipients serve as electron transport centers
- Key catalytic triplet (Glu368 - Arg256 - Tyr375) to participate in the dehydrogenation reaction
- Oxygen sensitive structure domain (Gly185 - Ser188) regulation of enzyme activity
Functions of ACADS
The core function of ACADS is to catalyze the β -oxidation of short-chain fatty acids and simultaneously participate in multiple metabolic regulatory processes.
| Function | Description |
| Metabolism of short-chain fatty acids | Specifically catalyze the α,β -dehydrogenation reaction of C4-C6 short-chain acyl-coA, initiating the first step of β -oxidation. |
| Electron transfer | Transfer electrons to electron transport flavoprotein (ETF) through FAD cofactors. |
| Energy supply | Each round of reaction generates 1 molecule of FADH2, contributing 1.5 ATP. |
| Metabolic regulation | Inhibit the fatty acid oxidation pathway through product accumulation feedback. |
| Metabolism of branched-chain amino acids | Participate in the degradation process of isoleucine and valine. |
| Disease association | Enzyme deficiency leads to ethylmalonic aciduria (SCADD). |
The substrate-specific curve of ACADS shows A typical "bell" feature, and it has the highest catalytic efficiency for butyryl-CoA (C4), which distinguishes it from other acyl-coA dehydrogenases.
Applications of ACADS and ACADS Antibody in Literature
1. Qian, Ze, et al. "DNA methylation of ACADS promotes immunogenic cell death in hepatocellular carcinoma." Cell & Bioscience 15.1 (2025): 3. https://doi.org/10.1186/s13578-024-01334-1
This article indicates that ACADS is lowly expressed in hepatocellular carcinoma (HCC) and highly methylated in the promoter region (such as the MS-2 site), and its overexpression can inhibit tumor progression and promote immunogenic cell death. The nomogram based on ACADS methylation can predict the survival rate of HCC patients, suggesting its potential as an immunotherapy target.
2. Matejka, Kerstin, et al. "Dynamic modelling of an ACADS genotype in fatty acid oxidation–Application of cellular models for the analysis of common genetic variants." Plos one 14.5 (2019): e0216110. https://doi.org/10.1371/journal.pone.0216110
This article indicates that down-regulation of ACADS gene expression (such as through shRNA knockdown or common variations) leads to elevated levels of short-chain (C4) and medium-long chain acylcarnitine. Lymphoblast and hepatocyte models confirmed that the regulation of ACADS expression can affect fatty acid oxidation metabolism, suggesting its association with the plasma acylcarnitine phenotype.
3. Lisyová, Jana, et al. "An unusually high frequency of SCAD deficiency caused by two pathogenic variants in the ACADS gene and its relationship to the ethnic structure in Slovakia." BMC medical genetics 19 (2018): 1-12. https://doi.org/10.1186/s12881-018-0566-0
This article indicates that neonatal screening in Slovakia has identified ACADS gene mutations (c.310_312delGAG and c.1138C>T) leading to short-chain acyl-coA dehydrogenase deficiency (SCADD), mainly manifested as elevated C4-acylcarnitine, with A high carrier rate in the ROM population. Long-term follow-up is required to assess the clinical impact.
4. Su, Zhiguang, et al. "Untangling HDL quantitative trait loci on mouse chromosome 5 and identifying Scarb1 and Acads as the underlying genes." Journal of Lipid Research 51.9 (2010): 2706-2713. https://doi.org/10.1194/jlr.M008110
This article indicates that on chromosome 5 of mice, the ACADS gene (Gly94Asp mutation) has been identified as the key gene of the HDL cholesterol quantitative trait locus Hdlq8. The difference in its protein level affects the HDL level. BALB/cBy mice have elevated HDL due to the deletion of ACADS.
5. Hornbak, Malene, et al. "The minor C-allele of rs2014355 in ACADS is associated with reduced insulin release following an oral glucose load." BMC Medical Genetics 12 (2011): 1-8.https://doi.org/10.1186/1471-2350-12-4
This article indicates that the C allele of the ACADS gene rs2014355 variant is associated with reduced insulin secretion after glucose stimulation, possibly by affecting fatty acid β -oxidation, but does not increase the risk of type 2 diabetes.
Creative Biolabs: ACADS Antibodies for Research
Creative Biolabs specializes in the production of high-quality ACADS antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Western Blot, Flow Cytometry, Immunohistochemistry, and other diagnostic methodologies.
- Custom ACADS Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ACADS antibodies, custom preparations, or technical support, please contact us.
Reference
- Lisyová, Jana, et al. "An unusually high frequency of SCAD deficiency caused by two pathogenic variants in the ACADS gene and its relationship to the ethnic structure in Slovakia." BMC medical genetics 19 (2018): 1-12. https://doi.org/10.1186/s12881-018-0566-0
Anti-ACADS antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ATP1A2 Recombinant Antibody (M7-PB-E9) (CBMAB-A4013-YC)

-
Mouse Anti-AKT1 Recombinant Antibody (V2-180546) (CBMAB-A2070-YC)

-
Mouse Anti-BRD3 Recombinant Antibody (CBYY-0801) (CBMAB-0804-YY)

-
Mouse Anti-dsRNA Recombinant Antibody (2) (CBMAB-D1807-YC)

-
Mouse Anti-C1QC Recombinant Antibody (CBFYC-0600) (CBMAB-C0654-FY)

-
Rabbit Anti-ENO2 Recombinant Antibody (BA0013) (CBMAB-0272CQ)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-AFM Recombinant Antibody (V2-634159) (CBMAB-AP185LY)

-
Mouse Anti-CEMIP Recombinant Antibody (3C12) (CBMAB-K0296-LY)

-
Mouse Anti-BIRC3 Recombinant Antibody (16E63) (CBMAB-C3367-LY)

-
Mouse Anti-CCND2 Recombinant Antibody (DCS-3) (CBMAB-G1318-LY)

-
Rat Anti-FABP3 Recombinant Antibody (CBXF-2299) (CBMAB-F1612-CQ)

-
Rabbit Anti-ALOX5AP Recombinant Antibody (CBXF-1219) (CBMAB-F0750-CQ)

-
Mouse Anti-ALB Recombinant Antibody (V2-55272) (CBMAB-H0819-FY)

-
Mouse Anti-CTNND1 Recombinant Antibody (CBFYC-2414) (CBMAB-C2487-FY)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Human Anti-SARS-CoV-2 Spike Recombinant Antibody (CBC05) (CBMAB-CR005LY)

-
Mouse Anti-dsDNA Recombinant Antibody (22) (CBMAB-AP1954LY)

-
Mouse Anti-FAS2 Monoclonal Antibody (1D4) (CBMAB-0071-CN)

-
Mouse Anti-CD1C Recombinant Antibody (L161) (CBMAB-C2173-CQ)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






