MX1 Antibodies
Background
MX1 gene encoding a GTP enzyme, made by the interferon induced mainly exist in the vertebrate immune cells and epithelial cells. This protein inhibits viral replication by hydrolyzing GTP and plays a crucial antiviral defense role in the innate immune system. Waterfowl animals particularly rely on the MX1 gene to maintain their viral defense capabilities because they are often exposed to highly variable avian influenza virus environments. This gene was first identified by Peter Staeheli's team in 1979 in the study of influenza virus resistance. Its unique trimer structure and GTPase activity mechanism were analyzed by cryo-electron microscopy in the 1990s. As a core effector molecule of the innate immune system, the dynamic conformational changes and viral ribonucleoprotein binding mechanism of MX1 protein continuously provide important theoretical models for the research and development of antiviral drugs.
Structure of MX1
The molecular weight of interferon-induced GTPase encoded by the MX1 gene is approximately 76 kDa. This value varies slightly among different species, mainly due to sequence-conformal variations in the GTP binding domain.
| Species | Human | Mouse | Domestic chicken | Zebrafish |
| Molecular Weight (kDa) | 76.0 | 75.8 | 76.2 | 75.5 |
| Primary Structural Differences | The carboxyl terminal contains a nuclear localization signal | The N-terminal is missing a spiral domain | Basic amino acids are inserted into the central domain | GTP enzyme activity area made highly conservative |
The MX1 protein is composed of 626 amino acids, and its three-dimensional structure presents a typical GTPase trimer conformation. The N-terminal of this protein carries a dynamic propeller domain, which is responsible for recognizing the viral ribonucleoprotein complex. The central GTP hydrolysis domain contains the conserved GXXGXGK motif, which is responsible for the hydrolysis of GTP and provides energy for conformational changes. The C-terminal domain forms a long α-helical bundle, mediating trimerization and interaction with macromolecular complexes. Its light yellow color (visible in denatured gels) is attributed to the change in ultraviolet absorption characteristics that occurs after binding to GTP molecules, which is directly related to the nucleotide binding properties of the protein.
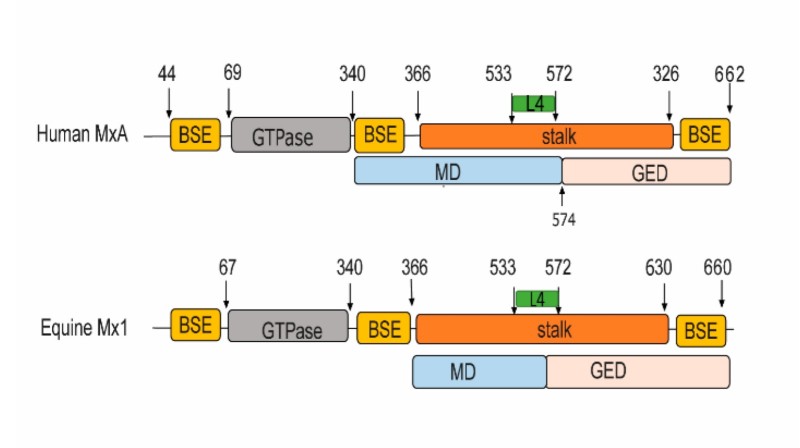 Fig. 1 Structure-based domain representation of human MxA and equine Mx1.1
Fig. 1 Structure-based domain representation of human MxA and equine Mx1.1
Key structural properties of MX1:
- Dynamic trimer GTPase structure
- Central viral ribonucleoprotein binding channels
- Conservative GTP hydrolyzed active pocket
- The carboxyl terminal regulates the domain
Functions of MX1
The core function of the protein encoded by the MX1 gene is to mediate the innate antiviral immune response of cells. As an interferon-induced dynamic GTPase, it specifically recognizes and inhibits the viral replication process in the cytoplasm.
| Function | Description |
| Recognition of viral ribonucleoprotein complexes | Directly by N end screw structure domain combination virus ribonucleoprotein (vRNP), blocking its process into the nucleus. |
| Gtpase-dependent virus inhibition | Hydrolyzing GTP triggers conformational changes, disrupts the function of viral polymerase and inhibits viral transcription and replication. |
| Interferon signaling pathway amplification | Interacting with the MAVS protein enhances the production of type I interferons, forming a positive feedback regulatory loop. |
| Cell-cytoplasmic shuttle regulation | By means of the nuclear localization signal (NLS), it enters the nucleus, captures the newly synthesized vRNP and prevents its cytosolic export. |
| Cross-species antiviral adaptation | The genes are driven by positive selection and show rapid evolutionary characteristics in different species, especially showing specific resistance to avian and mammalian influenza viruses. |
The GTP hydrolysis activity of MX1 protein shows allosteric regulation characteristics. Different from the linear dose-response of classical G proteins, the hydrolysis efficiency is significantly improved after the formation of the trimer. The expression level of this protein can increase by more than 20 times under the stimulation of interferon, forming the first line of antiviral defense in respiratory mucosal epithelial cells and immune cells. The variation at the 631st amino acid site (Asn→Asp) of the waterfowl MX1 protein has been confirmed to enhance the resistance to highly pathogenic avian influenza virus strains. This discovery provides a molecular target for transgenic disease-resistant breeding.
Applications of MX1 and MX1 Antibody in Literature
1. Jung, Hi Eun, Ji Eun Oh, and Heung Kyu Lee. "Cell-penetrating Mx1 enhances anti-viral resistance against mucosal influenza viral infection." Viruses 11.2 (2019): 109. https://doi.org/10.3390/v11020109
The article indicates that Mx1 is an antiviral protein that can inhibit the RNA transcription of influenza virus. In this study, polyarginine cell-penetrating peptide was fused with Mx1 to form MX1-9R. Experiments showed that it could be effectively taken up by cells, inhibit viral replication, and increase the survival rate of infected mice. It is expected to become a new strategy for anti-influenza.
2. Wang, Guisheng, et al. "MX1 and UBE2L6 are potential metaflammation gene targets in both diabetes and atherosclerosis." PeerJ 12 (2024): e16975. https://doi.org/10.7717/peerj.16975
This study found through a pig model of diabetes complicated with atherosclerosis that MX1 and UBE2L6 are key genes in the coexistence mechanism of the two diseases. They are significantly highly expressed in the lesion group and may affect the inflammatory response through the micronutrient metabolic pathway, suggesting that MX1 may serve as a potential biomarker for diabetes complicated with atherosclerosis.
3. Fatima, Urooj, et al. "Equine mx1 restricts influenza a virus replication by targeting at distinct site of its nucleoprotein." Viruses 11.12 (2019): 1114. https://doi.org/10.3390/v11121114
Studies have shown that adaptive mutations of NP (such as G34S and H52N) can lead to virus resistance to eqMx1. Therefore, eqMx1 is an important force driving the evolution of influenza virus nucleoproteins, and its drug-resistant amino acid sites can serve as key indicators for evaluating the virus's epidemic potential.
4. Schwab, Lara SU, et al. "Expression of a functional Mx1 protein is essential for the ability of RIG-I agonist prophylaxis to provide potent and long-lasting protection in a mouse model of influenza a virus infection." Viruses 14.7 (2022): 1547. https://doi.org/10.3390/v14071547
Through comparative experiments in this study, it was found that mice expressing functional Mx1 protein (B6.A2G-Mx1) could significantly induce the expression of interferon-stimulated genes (ISGs) after pretreatment with RIG-1 agonist 3pRNA, and exert a potent and long-lasting protective effect against influenza A virus (IAV) infection. Although mice with non-functional Mx1 can induce ISGs, their protective effect is limited, indicating that Mx1 is a key factor in the antiviral protection mediated by RIG-I agonists.
5. Nam, Ha, et al. "Elucidating the characteristics of Mx1 and resistance to influenza A virus subtype H1N1 in the newly developed KWM/Hym mice." Laboratory Animal Research 38.1 (2022): 28. https://doi.org/10.1186/s42826-022-00138-z
The article indicates that KWM/Hym inbred mice have significant resistance to influenza A virus due to multiple mutations in the Mx1 gene. All the mice survived after infection, with low viral load in their lungs and mild lesions. It is an ideal animal model for studying the mechanism of influenza virus.
Creative Biolabs: MX1 Antibodies for Research
Creative Biolabs specializes in the production of high-quality MX1 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom MXI Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our MX1 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Fatima, Urooj, et al. "Equine mx1 restricts influenza a virus replication by targeting at distinct site of its nucleoprotein." Viruses 11.12 (2019): 1114. https://doi.org/10.3390/v11121114
Anti-MX1 antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ABCA3 Recombinant Antibody (V2-178911) (CBMAB-A0145-YC)

-
Mouse Anti-BIRC3 Recombinant Antibody (315304) (CBMAB-1214-CN)

-
Mouse Anti-ATG5 Recombinant Antibody (9H197) (CBMAB-A3945-YC)

-
Mouse Anti-ATP1B3 Recombinant Antibody (1E9) (CBMAB-A4021-YC)

-
Mouse Anti-ADIPOR2 Recombinant Antibody (V2-179983) (CBMAB-A1369-YC)

-
Mouse Anti-APOA1 Monoclonal Antibody (CBFYR0637) (CBMAB-R0637-FY)

-
Mouse Anti-ACTG1 Recombinant Antibody (V2-179597) (CBMAB-A0916-YC)

-
Mouse Anti-BLK Recombinant Antibody (CBYY-0618) (CBMAB-0621-YY)

-
Mouse Anti-APCS Recombinant Antibody (CBYC-A663) (CBMAB-A3054-YC)

-
Mouse Anti-DDC Recombinant Antibody (8E8) (CBMAB-0992-YC)

-
Mouse Anti-CGAS Recombinant Antibody (CBFYM-0995) (CBMAB-M1146-FY)

-
Mouse Anti-ADGRE2 Recombinant Antibody (V2-261270) (CBMAB-C0813-LY)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Mouse Anti-CARD11 Recombinant Antibody (CBFYC-0811) (CBMAB-C0866-FY)

-
Rat Anti-EMCN Recombinant Antibody (28) (CBMAB-E0280-FY)

-
Rat Anti-CCR2 Recombinant Antibody (475301) (CBMAB-C1338-LY)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Mouse Anti-APOH Recombinant Antibody (4D9A4) (CBMAB-A3249-YC)

-
Mouse Anti-CTCF Recombinant Antibody (CBFYC-2371) (CBMAB-C2443-FY)

-
Mouse Anti-APP Recombinant Antibody (5C2A1) (CBMAB-A3314-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






