RHEB Antibodies
Background
RHEB is a highly conserved small molecule GTP-binding protein, mainly existing in the cytoplasm of mammals. This protein activates the mTORC1 pathway by sensing nutritional signals and energy states, thereby regulating key biological processes such as cell growth, proliferation and autophagy. Research has found that RHEB plays a significant role in neurodevelopment and tumorigenesis, and its functional abnormalities are closely related to epilepsy, autism spectrum disorders, and various types of cancer. This gene was first discovered in the rat brain in 1994, and the study of its three-dimensional structure revealed a unique GTPase activation mechanism. As a core regulatory factor of the mTOR signaling pathway, RHEB has become an important molecular target for the study of metabolic diseases and targeted anti-cancer therapy.
Structure of RHEB
RHEB is a small molecule GTPase with a molecular weight of approximately 20.6 kDa. There are slight differences in this molecular weight among different species, mainly due to minor changes in the amino acid sequence.
| Species | Human | Mouse | Rat | Fruit fly | Yeast |
| Molecular Weight (kDa) | 20.6 | 20.5 | 20.5 | 20.8 | 21.0 |
| Primary Structural Differences | Highly conserved G domain | 93% homologous to human origin | Highly similar to the human sequence | Has a unique loop region insertion | The domains of GTPase are similar |
The RHEB protein is composed of 184 amino acids and presents a typical spherical conformation of a GTP-binding protein. Its three-dimensional structure is mainly composed of five β -folds and six α -helices, which together form the GTP/GDP binding pocket. The effector domains of this protein, especially the Switch I and Switch II regions, undergo conformational changes after binding to GTP, thereby being able to specifically activate the mTORC1 signaling pathway. The arginine residue at position 64 is crucial for the hydrolysis of GTP, while the asparagine residue at position 32 is involved in the recognition and conversion between GTP and GDP.
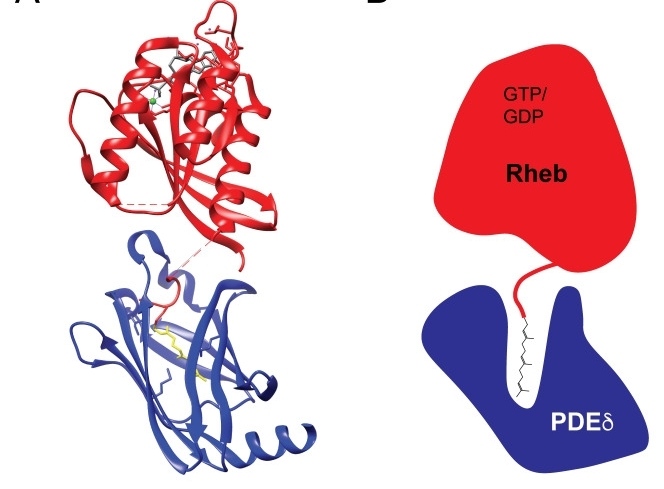 Fig. 1 Rheb C-terminal farnesylation allows for interactions with PDEδ.1
Fig. 1 Rheb C-terminal farnesylation allows for interactions with PDEδ.1
Key structural properties of RHEB:
- Typical globular domains of the Ras superfamily of gtpases
- Highly conservative GTP/GDP binding pocket (G domain)
- Switch I and Switch II conformational variable regions are responsible for molecular switching function
- Effector binding specificity ring (residues 64-67) to identify mTORC1 signaling pathways
- C terminal CAAX motif mediated cell membrane localization and signal transduction
Functions of RHEB
The core function of RHEB protein is to serve as a key regulatory factor for cell growth and metabolism. It mainly functions by activating the mTORC1 signaling pathway, and is also involved in various physiological processes such as autophagy regulation, energy sensing, and cellular stress response.
| Function | Description |
| Activation of the mTORC1 pathway | RHEB directly binds to and activates the mTORC1 complex in the GTP-bound state, promoting protein synthesis and cell growth. |
| Regulation of cell proliferation | Regulating mTOR signaling promotes cell cycle progression and influences tissue development and regeneration processes. |
| Inhibition of autophagy | Activated mTORC1 inhibits the formation of autophagosomes and maintains cell anabolism when nutrients are adequate. |
| Energy state sensing | Sense the ATP level and nutritional status within cells, and coordinate the balance between anabolism and catabolism. |
| Regulation of stress response | Under hypoxic or energy stress conditions, RHEB activity is regulated through the TSC complex to help cells adapt to environmental changes. |
The activation cycle of RhegTPase exhibits typical binary switch characteristics. Compared with other members of the Ras superfamily, its specific recognition and continuous activation ability for mTORC1 are significantly stronger, making it a key molecular hub for cells to sense microenvironmental signals and coordinate anabrometabolism.
Applications of RHEB and RHEB Antibody in Literature
1. Wang, Xinyue, et al. "A novel rabbit anti-myoglobin monoclonal antibody's potential application in rhabdomyolysis associated acute kidney injury." International Journal of Molecular Sciences 24.9 (2023): 7822. https://doi.org/10.3389/fcell.2022.808140
Research has found that Rheb regulates liver lipid secretion by promoting mitochondrial ATP production. The deficiency of Rheb can cause spontaneous fatty liver, while overexpression alleviates diet-induced hepatic steatosis. This function is independent of the lipid synthesis promoting effect of mTORC1.
2. Groenewoud, Marlous J., and Fried JT Zwartkruis. "Rheb and mammalian target of rapamycin in mitochondrial homoeostasis." Open biology 3.12 (2013): 130185. https://doi.org/https:/10.1098/rsob.130185
Studies have found that the Rheb-mTORC1 signaling pathway affects mitochondrial biosynthesis and mitochondrial autophagy by regulating transcription factors such as HIF1α and YY1/PGC-1α, thereby maintaining mitochondrial homeostasis. This pathway forms a feedback regulatory circuit with mitochondrial reactive oxygen species signals.
3. He, aobo, et al. "Lysosomal EGFR acts as a Rheb-GEF independent of its kinase activity to activate mTORC1." Cell Research (2025): 1-13. https://doi.org/10.1038/s41422-025-01110-x
Research has found that EGFR can act as a guanylate exchange factor (GEF) for Rheb, directly activating Rheb and promoting the mTORC1 signaling pathway. Its GEF function depends on the E804 site and is independent of kinase activity, providing a new strategy for targeting EGFR-mutated tumors.
4. Brittany, Angarola, and Shawn M. Ferguson. "Coordination of Rheb lysosomal membrane interactions with mTORC1 activation." F1000Research 9 (2020). https://doi.org/10.12688/f1000research.22367.1
The article indicates that Rheb is located on the lysosomal membrane through C-terminal farinylation, and its weak membrane interaction characteristics are crucial for the effective activation of mTORC1. This localization mechanism is achieved through the interaction with PDEδ/Arl2, which is distinct from other Ras superfamily proteins and demonstrates species diversity.
5. Deng, L. U., et al. "Ubiquitination of Rheb governs growth factor-induced mTORC1 activation." Cell research 29.2 (2019): 136-150. https://doi.org/10.1038/s41422-018-0120-9
The article indicates that RNF152-mediated Rheb ubiquitination promotes its binding to the TSC complex and inhibits the activity of mTORC1. Under the action of AKT, USP4 ubiquitinates Rheb and activates the mTORC1 signaling pathway. This ubiquitination regulatory mechanism affects tumor growth.
Creative Biolabs: RHEB Antibodies for Research
Creative Biolabs specializes in the production of high-quality myoglobin antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom RHEB Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our RHEB antibodies, custom preparations, or technical support, contact us at email.
Reference
- Brittany, Angarola, and Shawn M. Ferguson. "Coordination of Rheb lysosomal membrane interactions with mTORC1 activation." F1000Research 9 (2020). https://doi.org/10.12688/f1000research.22367.1
Anti-RHEB antibodies
 Loading...
Loading...
Hot products 
-
Rabbit Anti-Acetyl-Histone H3 (Lys36) Recombinant Antibody (V2-623395) (CBMAB-CP0994-LY)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-ALB Recombinant Antibody (V2-180650) (CBMAB-A2186-YC)

-
Rabbit Anti-CCL5 Recombinant Antibody (R0437) (CBMAB-R0437-CN)

-
Mouse Anti-DMPK Recombinant Antibody (CBYCD-324) (CBMAB-D1200-YC)

-
Mouse Anti-BZLF1 Recombinant Antibody (BZ.1) (CBMAB-AP705LY)

-
Mouse Anti-CD8 Recombinant Antibody (C1083) (CBMAB-C1083-LY)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503416) (CBMAB-V208-1402-FY)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Mouse Anti-FLI1 Recombinant Antibody (CBXF-0733) (CBMAB-F0435-CQ)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-0790) (CBMAB-0793-YY)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Mouse Anti-ADAM12 Recombinant Antibody (V2-179752) (CBMAB-A1114-YC)

-
Mouse Anti-AK4 Recombinant Antibody (V2-180419) (CBMAB-A1891-YC)

-
Mouse Anti-CCL18 Recombinant Antibody (64507) (CBMAB-C7910-LY)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-1728) (CBMAB-2077-YY)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Mouse Anti-FN1 Monoclonal Antibody (D6) (CBMAB-1240CQ)

-
Mouse Anti-DLG1 Monolconal Antibody (4F3) (CBMAB-0225-CN)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








