TIPIN Antibodies
Background
TIPIN protein, as a key component of the DNA replication fork complex in the cell nucleus, is mainly present in proliferating cells of eukaryotes. This protein forms a heterodimer with TIMELESS, jointly stabilizing the DNA replication fork structure and coordinating the linkage between replication and cell cycle checkpoints, which plays a core role in maintaining genomic stability. Under DNA replication stress conditions, TIPIN achieves replication fork protection by mediating the activation of the ATR-CHK1 signaling pathway. The loss of its function will lead to replication fork collapse and chromosomal aberration. This gene was first identified by the Japanese scholar Yoshizawa's team in 2005. Its three-dimensional structure was analyzed by cryo-electron microscopy in 2016, revealing the synergistic mechanism of the TIMELESS TIPIN complex and DNA polymerase ε. As the core hub of the DNA replication guarantee system, TIPIN has become an important molecular target for studying genomic stability, tumorigenesis and targeted therapy.
Structure of TIPIN
The molecular weight of TIPIN protein is approximately 39 kDa. Its molecular weight shows a high degree of conservation across different species, which is attributed to the core functional constraints of this protein in DNA replication fork protection.
| Species | Human | Mouse | African clawed toad | Fruit fly | Yeast |
| Molecular Weight (kDa) | 39.2 | 38.9 | 39.5 | 37.8 | 33.1 |
| Primary Structural Differences | Containing the conserved coiled-coil domain | The C-terminal sequence has slight variations | Retain the functional domain of human homologues | Simplified copy cross association domain | Only the core dimerization module is included |
The TIPIN protein is composed of 349 amino acids, and its primary structure includes the N-terminal dimerization domain and the C-terminal nucleic acid binding region. This protein forms a stable heterodimer with TIMELESS through helical-helical interactions, and this complex binds to single-stranded DNA through its unique basic amino acid cluster. Its secondary structure is a groove structure mainly formed by α -helices, providing a bonding interface for the replication fork components. The C-terminal domain of TIPIN contains typical nuclear localization signals, while the N-terminal coiled-coil motif directly participates in the transduction of ATR checkpoint signals. This precise structural arrangement ensures the stability of the genome during DNA replication.
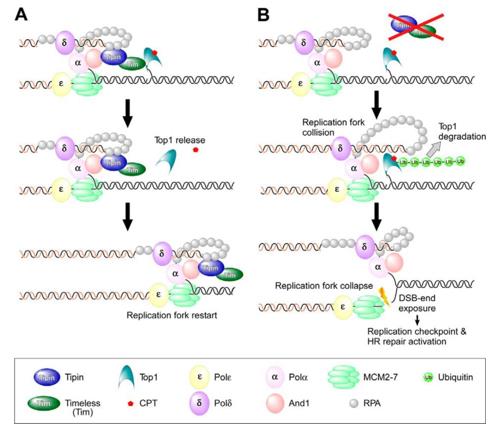 Fig. 1 Model of Tipin-mediated protection of the replication fork following CPT treatment.1
Fig. 1 Model of Tipin-mediated protection of the replication fork following CPT treatment.1
Key structural properties of TIPIN:
- Stable coiled-coil dimerization domains are formed with TIMELESS
- Conserved basic amino acid clusters form the DNA binding interface
- Nuclear localization signals at the C-terminal regulate intracellular localization
- Replication forks protection die body combined with ATR kinase directly
Functions of TIPIN
The core function of the TIPIN protein is to maintain the stability and integrity of DNA replication forks. In addition, it is also involved in a variety of key cellular processes such as the regulation of cell cycle checkpoints and replication stress responses.
| Function | Description |
| Copy fork stability | Forms a heterodimer with TIMELESS to physically stabilize the replication fork structure and prevent its collapse. |
| DNA damage response | Recruit the ATR-ATRIP kinase complex to the arrest replication fork to initiate the checkpoint signaling pathway. |
| Replication stress relief | Coordinate the replication and repair processes to prevent the occurrence of DNA double-strand breaks. |
| Cell cycle regulation | By regulating the activity of CDC7 kinase, it affects the initiation timing of DNA replication. |
| Maintenance of genomic integrity | Ensure the faithful completion of DNA replication and prevent chromosomal aberrations and tumor occurrence. |
The TIPIN-TIMELESS complex forms a structural platform at the replication fork. The loss of its function directly leads to the collapse of the replication fork and DNA double-strand breaks. This mechanism shows significant abnormalities in various cancers.
Applications of TIPIN and TIPIN Antibody in Literature
1. Abe, Takuya, et al. "TIPIN is essential for chromosome stability and cell viability in BRCA1-deficient cells." Biochemical and Biophysical Research Communications 752 (2025): 151467. https://doi.org/10.1016/j.bbrc.2025.151467
Research has found that BRCA1-deficient breast cancer has a "synthetic lethal" effect with the replication protein TIPIN. The mechanism lies in that the DNA damage caused by TIPIN deletion needs to be repaired by BRCA1-mediated homologous recombination.
2. Prorok, Paulina, Eva Wolf, and M. Cristina Cardoso. "Timeless–Tipin interactions with MCM and RPA mediate DNA replication stress response." Frontiers in Cell and Developmental Biology 12 (2024): 1346534. https://doi.org/10.3389/fcell.2024.1346534
Studies have shown that the Timeless and Tipin complex is the core guardian component of DNA replicas. Whether in normal replication or under pressure, it always accompanies the helicase to move forward, ensuring the stability of the replication process.
3. Hosono, Yoshifumi, et al. "Tipin functions in the protection against topoisomerase I inhibitor." Journal of Biological Chemistry 289.16 (2014): 11374-11384. https://doi.org/10.1074/jbc.M113.531707
Research reveals that cells lacking TIPIN are highly sensitive to the anti-cancer drug CPT. Because its replication fork is more likely to collide with the DNA-topoisomerase I complex captured by CPT, it leads to the obstruction of DNA synthesis and the degradation of the complex.
4. Chakraborty, Abhijit, et al. "Knock-down of the TIM/TIPIN complex promotes apoptosis in melanoma cells." Oncotarget 11.20 (2020): 1846. https://doi.org/10.18632/oncotarget.27572
Studies have found that the TIM-TIPIN complex is overexpressed in melanoma and is associated with a poor prognosis. Knocking down this complex can inhibit tumor growth by inducing DNA damage and apoptosis, suggesting its potential as a new therapeutic target.
5. Yisui, et al. "TIMELESS‐TIPIN and UBXN‐3 promote replisome disassembly during DNA replication termination in Caenorhabditis elegans." The EMBO Journal 40.17 (2021): e108053. https://doi.org/10.15252/embj.2021108053
Research reveals that the TIMELESS TIPIN complex is responsible for recruiting CUL-2 ubiquitin ligases when DNA replication is terminated, thereby initiating the ubiquitination and depolymerization of CMG helicases. This mechanism provides new ideas for anti-cancer treatment.
Creative Biolabs: TIPIN Antibodies for Research
Creative Biolabs specializes in the production of high-quality TIPIN antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom TIPIN Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our TIPIN antibodies, custom preparations, or technical support, contact us at email.
Reference
- Hosono, Yoshifumi, et al. "Tipin functions in the protection against topoisomerase I inhibitor." Journal of Biological Chemistry 289.16 (2014): 11374-11384. https://doi.org/10.1074/jbc.M113.531707
Anti-TIPIN antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-CTNND1 Recombinant Antibody (CBFYC-2414) (CBMAB-C2487-FY)

-
Mouse Anti-ENO1 Recombinant Antibody (CBYC-A950) (CBMAB-A4388-YC)

-
Mouse Anti-ADGRE5 Recombinant Antibody (V2-360335) (CBMAB-C2088-CQ)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-0790) (CBMAB-0793-YY)

-
Rat Anti-CCR2 Recombinant Antibody (475301) (CBMAB-C1338-LY)

-
Mouse Anti-CAT Recombinant Antibody (724810) (CBMAB-C8431-LY)

-
Mouse Anti-EIF4G1 Recombinant Antibody (2A9) (CBMAB-A2544-LY)

-
Mouse Anti-CD247 Recombinant Antibody (6B10.2) (CBMAB-C1583-YY)

-
Mouse Anti-BSN Recombinant Antibody (219E1) (CBMAB-1228-CN)

-
Mouse Anti-BCL6 Recombinant Antibody (CBYY-0442) (CBMAB-0445-YY)

-
Mouse Anti-CECR2 Recombinant Antibody (CBWJC-2465) (CBMAB-C3533WJ)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-COL12A1 Recombinant Antibody (CBYY-C3117) (CBMAB-C4560-YY)

-
Mouse Anti-ALB Recombinant Antibody (V2-55272) (CBMAB-H0819-FY)

-
Mouse Anti-ATP1B1 Recombinant Antibody (E4) (CBMAB-0463-LY)

-
Rabbit Anti-AKT3 Recombinant Antibody (V2-12567) (CBMAB-1057-CN)

-
Rabbit Anti-ATF4 Recombinant Antibody (D4B8) (CBMAB-A3872-YC)

-
Mouse Anti-CCNH Recombinant Antibody (CBFYC-1054) (CBMAB-C1111-FY)

-
Mouse Anti-CASP8 Recombinant Antibody (CBYY-C0987) (CBMAB-C2424-YY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






