NANOG Antibodies
Background
NANOG is a key transcription factor protein, mainly present in embryonic stem cells and pluripotent stem cells of mammals. This gene plays a core role in embryonic development and cell reprogramming by maintaining the self-renewal ability and pluripotency of stem cells. It was first discovered in mouse embryonic stem cells by Japanese scholars in 2003. Its name is derived from "Tir Na Nog" (the Land of Youth), which symbolizes eternal youth in Irish mythology. The three-dimensional structure study of NANOG protein has revealed the unique mechanism of its DNA-binding domain, providing an important foundation for stem cell biology and regenerative medicine research. As a core component of the pluripotency regulatory network, NANOG works in synergy with factors such as OCT4 and SOX2. The precise regulation of its expression level is crucial for maintaining the characteristics of stem cells. The related findings have brought new possibilities to disease modeling and cell therapy.
Structure of NANOG
NANOG is a key transcription factor protein with a molecular weight of approximately 35 kDa. There are slight differences in its molecular weight among different species, mainly due to minor changes in amino acid sequences.
| Species | Human | Mouse | Rat | Pig |
| Molecular Weight (kDa) | 34.8 | 35.2 | 35.0 | 34.9 |
| Primary Structural Differences | Conserved DNA binding domain with homologous box structure | Highly similar to humans, but slightly different in the C-end | Core functional areas are highly conservative | Similar to primate NANOG |
The NANOG protein is composed of approximately 305 amino acids and has a typical homeobox domain, which enables it to specifically bind to DNA-regulated target genes. Its three-dimensional structure presents a typical helix-turn-helix conformation, in which the key amino acids at positions 152-212 form the DNA binding core region. NANOG interacts with other stem cell factors (such as OCT4 and SOX2) through its N-terminal transcriptional activation domain and C-terminal regulatory domain to jointly maintain stem cell pluripotency. The conserved structure of this protein enables it to maintain functional stability during evolution, while there are subtle differences among species to adapt to different regulatory requirements.
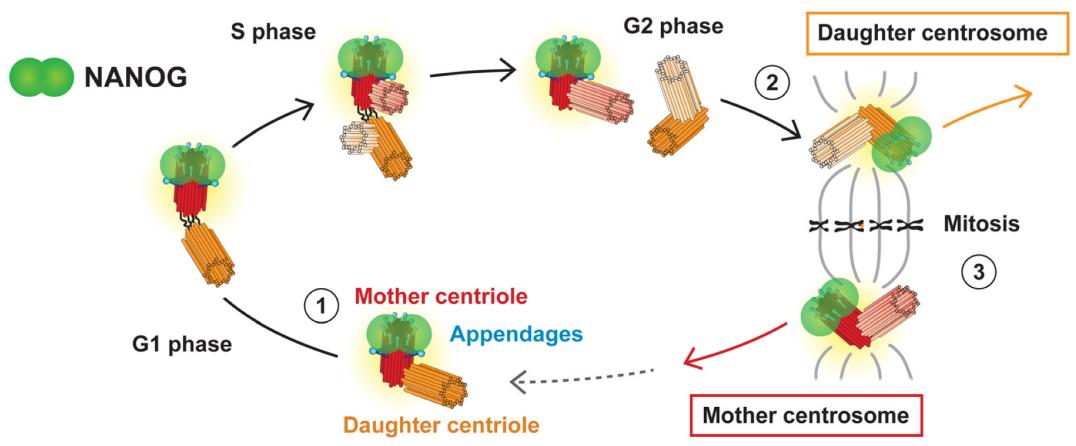 Fig. 1 Study on the Dynamic Localization Characteristics of Centrosomes in the Cell Cycle of NANOG/NANOGP8.1
Fig. 1 Study on the Dynamic Localization Characteristics of Centrosomes in the Cell Cycle of NANOG/NANOGP8.1
Key structural properties of NANOG:
- Conserved Homeobox domain
- N-terminal transcriptional activation domain
- C-end regulation area
- Key tryptophan (W) and histidine (H) residues
- Phosphorylation site
Functions of NANOG
The core function of the NANOG gene is to maintain the pluripotency and self-renewal ability of stem cells, and it also plays a key role in cell reprogramming and early embryonic development.
| Function | Description |
| Maintain pluripotency | By activating pluripotent related genes (such as OCT4, SOX2) and inhibiting differentiation genes, it ensures that stem cells remain undifferentiated. |
| Regulation of embryonic development | Within the blastocyst cell mass (ICM) increased, the early embryonic survival and normal development is very important. |
| Cell reprogramming | Somatic cells can be induced into pluripotent stem cells (iPSCs) by cooperating with Yamanaka factors (OCT4, SOX2, KLF4). |
| Anti-differentiation effect | Inhibit the genes related to mesoderm and endoderm differentiation to maintain the plasticity of stem cells. |
| Epigenetic regulation | Regulate chromatin opening status and promote pluripotent gene expression by influencing histone modifications (e.g. H3K27me3, H3K4me3). |
The expression level of NANOG shows dynamic fluctuations, and its precise regulation is crucial for the fate determination of stem cells - overexpression may lead to tumor-like growth, while underexpression promotes cell differentiation. This characteristic makes it an important target for research in regenerative medicine and disease treatment.
Applications of NANOG and NANOG Antibody in Literature
1. Cardenas, Ryan P., et al. "NANOG controls testicular germ cell tumour stemness through regulation of MIR9-2." Stem Cell Research & Therapy 15.1 (2024): 128. https://doi.org/10.1186/s13287-024-03724-1
The article indicates that NANOG affects the dry and malignant progression of testicular germ cell tumors (TGCT) by regulating MIR9-2-5p. Low expression of MIR9-2-5p promotes tumor progression, while its overexpression can inhibit proliferation, invasion and cisplatin resistance, suggesting its therapeutic potential as a novel tumor suppressor factor.
2. Saito, Mikako. "Novel roles of Nanog in cancer cells and their extracellular vesicles." Cells 11.23 (2022): 3881. https://doi.org/10.3390/cells11233881
Research has found that melanoma extracellular vesicles (EVs) overexpressing NANOG unexpectedly have an inhibitory effect on metastasis, revealing a new role of NANOG in regulating the function of tumor EVs. Although NANOG promotes the malignant progression of cancer in most cases, this discovery provides new ideas for the development of EV-based tumor vaccines.
3. Mikulenkova, Erika, et al. "NANOG/NANOGP8 localizes at the centrosome and is spatiotemporally associated with centriole maturation." Cells 9.3 (2020): 692. https://doi.org/10.3390/cells9030692
Research has found that NANOG is a key transcription factor regulating the pluripotency of stem cells and maintaining the characteristics of cancer stem cells in various tumors. Research has found that NANOG and its pseudogene NANOGP8 exhibit abnormal centrosome localization in different cells, providing new clues for revealing their non-classical functions.
4. Allègre, Nicolas, et al. "NANOG initiates epiblast fate through the coordination of pluripotency genes expression." Nature Communications 13.1 (2022): 3550. https://doi.org/10.1038/s41467-022-30858-8
Research has found that NANOG expression is an independent prognostic factor for esophageal adenocarcinoma, and the overall survival of positive patients is significantly shortened (p=0.002). This finding supports NANOG as a stratification marker for high-risk patients and a potential therapeutic target, especially for the patient population that has not received neoadjuvant therapy (HR=1.40).
5. Son, Sung Wook, et al. "NANOG confers resistance to complement-dependent cytotoxicity in immune-edited tumor cells through up-regulating CD59." Scientific reports 12.1 (2022): 8652. https://doi.org/10.1038/s41598-022-12692-6
Research has found that NANOG mediates cross-resistance of tumor cells to the complement system by up-regulating CD59. Targeting NANOG can enhance the complement-dependent cytotoxicity of trastuzumab, providing a new strategy for the treatment of immune-edited tumors.
Creative Biolabs: NANOG Antibodies for Research
Creative Biolabs specializes in the production of high-quality NANOG antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom NANOG Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our NANOG antibodies, custom preparations, or technical support, contact us at email.
Reference
- Mikulenkova, Erika, et al. "NANOG/NANOGP8 localizes at the centrosome and is spatiotemporally associated with centriole maturation." Cells 9.3 (2020): 692. https://doi.org/10.3390/cells9030692
Anti-NANOG antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-ABL2 Recombinant Antibody (V2-179121) (CBMAB-A0364-YC)

-
Mouse Anti-ADRB2 Recombinant Antibody (V2-180026) (CBMAB-A1420-YC)

-
Mouse Anti-ACTB Recombinant Antibody (V2-179553) (CBMAB-A0870-YC)

-
Mouse Anti-CD46 Recombinant Antibody (CBFYC-0076) (CBMAB-C0085-FY)

-
Rabbit Anti-Acetyl-Histone H3 (Lys36) Recombinant Antibody (V2-623395) (CBMAB-CP0994-LY)

-
Mouse Anti-CECR2 Recombinant Antibody (CBWJC-2465) (CBMAB-C3533WJ)

-
Mouse Anti-DLG1 Monolconal Antibody (4F3) (CBMAB-0225-CN)

-
Mouse Anti-BMI1 Recombinant Antibody (CBYC-P026) (CBMAB-P0108-YC)

-
Mouse Anti-A2M Recombinant Antibody (V2-178822) (CBMAB-A0036-YC)

-
Mouse Anti-ATP1A2 Recombinant Antibody (M7-PB-E9) (CBMAB-A4013-YC)

-
Mouse Anti-CASP7 Recombinant Antibody (10-01-62) (CBMAB-C2005-LY)

-
Mouse Anti-AAV-5 Recombinant Antibody (V2-503416) (CBMAB-V208-1402-FY)

-
Rat Anti-FABP3 Recombinant Antibody (CBXF-2299) (CBMAB-F1612-CQ)

-
Mouse Anti-NSUN6 Recombinant Antibody (D-5) (CBMAB-N3674-WJ)

-
Rat Anti-ADAM10 Recombinant Antibody (V2-179741) (CBMAB-A1103-YC)

-
Mouse Anti-CD24 Recombinant Antibody (ALB9) (CBMAB-0176CQ)

-
Mouse Anti-CD2AP Recombinant Antibody (BR083) (CBMAB-BR083LY)

-
Mouse Anti-APP Recombinant Antibody (DE2B4) (CBMAB-1122-CN)

-
Mouse Anti-AGO2 Recombinant Antibody (V2-634169) (CBMAB-AP203LY)

-
Mouse Anti-ACVR1C Recombinant Antibody (V2-179685) (CBMAB-A1041-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








