PIGO Antibodies
Background
The PIGO gene encodes a phosphatidylinositol glycan anchored biosynthetic protein, which is located on the endoplasmic reticulum membrane and mainly participates in the biosynthesis process of glycosylphosphatidylinositol anchors. Its encoded protein ensures that multiple membrane proteins can be correctly anchored to the cell surface by catalyzing the covalent binding of GPI anchors to target proteins, thereby maintaining key physiological functions such as cell signal transduction and immune response. The defect of this gene is closely related to the autosomal recessive genetic disorder - hyperphosphatase syndrome with intellectual disability syndrome type 4, which is clinically manifested as abnormal development of the nervous system and imbalance of bone metabolism. As a core member of the GPI-anchored synthetic pathway, the molecular mechanism research of the PIGO gene not only reveals the important rules of post-translational modification of proteins, but also provides key targets for the diagnosis and treatment of congenital glycosylation diseases.
Structure of PIGO
The molecular weight of the protein encoded by the PIGO gene is approximately 65 kDa. This protein is composed of 579 amino acids, and its structure mainly includes the N-terminal signal peptide, transmembrane domain and C-terminal catalytic functional region. The sequence of this protein is highly conserved among different species, especially near the catalytic active site.
| Species | Human | Mouse | Zebrafish | Fruit fly |
| Molecular Weight (kDa) | 65.2 | 64.8 | 63.5 | 61.3 |
| Primary Structural Differences | Complete catalytic domain | 92% homology with humans | The core domain is conservative | Only retain the basic functional modules |
The catalytic domain of this protein contains conserved basic residues, which are responsible for recognizing GPI precursors and catalyzing their connection with target proteins. The transmembrane helix ensures its stable anchoring on the coarse endoplasmic reticulum membrane, while the N-terminal signal peptide guides it into the secretory pathway. This structural feature enables it to efficiently participate in the biosynthesis process of GPI anchors.
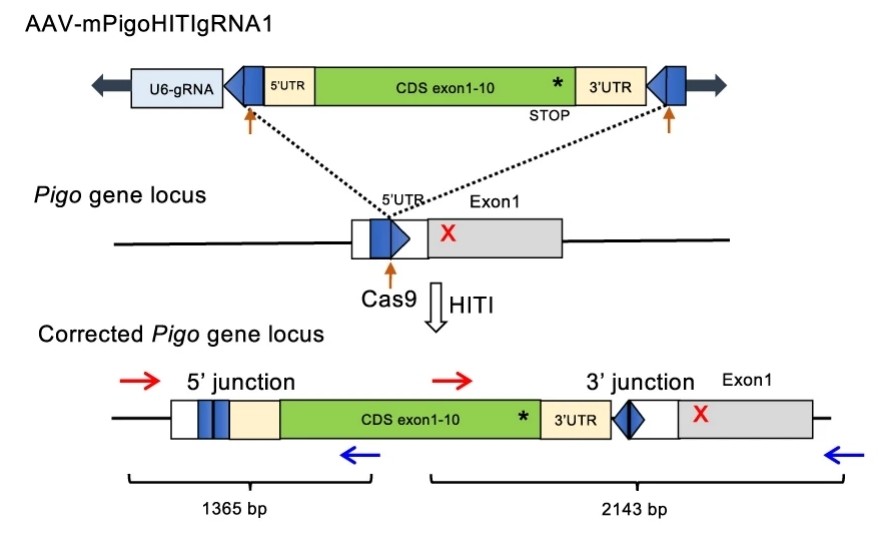 Fig. 1 Schematic representation of the Pigo KI allele b (T130N, c.389 C > A) genome editing by a HITI donor.1
Fig. 1 Schematic representation of the Pigo KI allele b (T130N, c.389 C > A) genome editing by a HITI donor.1
Key structural properties of PIGO:
- Er membranes anchor transmembrane structures
- Conservative alkaline catalytic active center
- GPI substrate-specific binding domains
Functions of PIGO
The core function of PIGO protein is to catalyze the biosynthesis of glycosylphosphatidylinositol anchors. In addition, it is also involved in the regulation of various cellular physiological processes.
| Function | Description |
| Gpi-anchored synthesis | Catalyze the covalent connection between GPI anchors and the C-terminal of proteins in the endoplasmic reticulum to complete post-translational modification. |
| Membrane protein localization | Ensure that multiple membrane proteins can be correctly anchored to the cell surface to maintain their physiological functions. |
| Immune regulation | By anchoring the complement regulatory proteins, immune factors such as involved in the immune response regulation. |
| Neural development | Ensure the correct localization of membrane proteins related to neural development and support the normal development of the nervous system. |
| Regulation of enzyme activity | Influence of a variety of membrane bound enzymes activity, participate in physiological processes such as bone metabolism. |
The catalytic efficiency of this protein is strictly regulated by its basic active center. This characteristic enables it to precisely recognize different GPI precursor structures and ensure specific modification of various substrate proteins. This mechanism is crucial for maintaining the normal function of the cell surface proteome.
Applications of PIGO and PIGO Antibody in Literature
1. Wang, Xinyue, et al. "A novel rabbit anti-myoglobin monoclonal antibody's potential application in rhabdomyolysis associated acute kidney injury." International Journal of Molecular Sciences 24.9 (2023): 7822. https://doi.org/10.1186/s13023-017-0654-9
In this study, through whole exome sequencing, two novel PIGO gene variations were identified in a boy presenting with deformities, psychomotor disorders, epilepsy, palmoplantar keratosis, hyperphosphatinemia and abnormal platelet function. The skin manifestations and platelet abnormalities of the child patients are features that have not been reported in previous cases of PIGO deficiency.
2. Starosta, Rodrigo Tzovenos, et al. "PIGO‐CDG: A case study with a new genotype, expansion of the phenotype, literature review, and nosological considerations." JIMD reports 64.6 (2023): 424-433. https://doi.org/10.1002/jmd2.12396
This research report presents a case of congenital glycosylation disorder of PIGO (PIGO-CDG) caused by a novel PIGO gene variation. In addition to the typical Mabry syndrome phenotype, the child also presented with new features such as intermittent hypoglycemia, severe diarrhea and osteoporosis, which may expand the known clinical manifestations of this disease. It is suggested that the disease be uniformly named PIGO-CDG.
3. Kuwayama, Ryoko, et al. "Establishment of mouse model of inherited PIGO deficiency and therapeutic potential of AAV-based gene therapy." Nature Communications 13.1 (2022): 3107. https://doi.org/10.1038/s41467-022-30847-x
This study successfully constructed a hereditary GPI-deficient mouse model caused by PIGO gene mutations and developed an effective gene therapy. Through AAV-mediated gene therapy, the neurophenotype and growth defects of mice were significantly improved, opening up a new path for curing this disease.
4. Dellepiane, Francesco, et al. "Two Different Brain Injury Patterns Associated with Compound Heterozygosis of the PIGO Gene in a Term Newborn: A Case Report." Biomedicines 12.12 (2024): 2779. https://doi.org/10.3390/biomedicines12122779
This study reports a case of a male newborn carrying a novel compound heterozygous PIGO gene variation. The clinical manifestations of the child patient were severe, including encephalopathy, refractory epilepsy and gastrointestinal abnormalities. For the first time, it was found that the brain MRI presented a new pattern of extensive cortical and subcortical involvement that had not been reported before. This case expands the neuroimaging phenotypic spectrum of PIGO deficiency.
5. Nakamura, Jun, et al. "Detection of PIGO-deficient cells using proaerolysin: a valuable tool to investigate mechanisms of mutagenesis in the DT40 cell system." PLoS One 7.3 (2012): e33563. https://doi.org/10.1371/journal.pone.0033563
In this study, a novel mutation detection method based on PA selection was established in the DT40 cell line for detecting GPI-anchored protein defects. This study took the PIG-O gene (located on the Z chromosome of poultry) as the key reporter gene and found that approximately 55% of the mutation sites after MMS mutagenesis were A:T, and mainly A→T transmutation. This method provides a new tool for studying the mechanism of action of mutagens.
Creative Biolabs: PIGO Antibodies for Research
Creative Biolabs specializes in the production of high-quality PIGO antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom PIGO Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our PIGO antibodies, custom preparations, or technical support, contact us at email.
Reference
- Kuwayama, Ryoko, et al. "Establishment of mouse model of inherited PIGO deficiency and therapeutic potential of AAV-based gene therapy." Nature Communications 13.1 (2022): 3107. https://doi.org/10.1038/s41467-022-30847-x
Anti-PIGO antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-C5B-9 Recombinant Antibody (CBFYA-0216) (CBMAB-X0304-FY)

-
Mouse Anti-FAS2 Monoclonal Antibody (1D4) (CBMAB-0071-CN)

-
Mouse Anti-CCN2 Recombinant Antibody (CBFYC-2383) (CBMAB-C2456-FY)

-
Rat Anti-AChR Recombinant Antibody (V2-12500) (CBMAB-0990-CN)

-
Rat Anti-EMCN Recombinant Antibody (28) (CBMAB-E0280-FY)

-
Mouse Anti-CTNND1 Recombinant Antibody (CBFYC-2414) (CBMAB-C2487-FY)

-
Mouse Anti-ELAVL4 Recombinant Antibody (6B9) (CBMAB-1132-YC)

-
Rat Anti-(1-5)-α-L-Arabinan Recombinant Antibody (V2-501861) (CBMAB-XB0003-YC)

-
Mouse Anti-EPO Recombinant Antibody (CBFYR0196) (CBMAB-R0196-FY)

-
Mouse Anti-CD24 Recombinant Antibody (HIS50) (CBMAB-C10123-LY)

-
Mouse Anti-CD46 Recombinant Antibody (CBFYC-0076) (CBMAB-C0085-FY)

-
Mouse Anti-ENPP1 Recombinant Antibody (CBFYE-0159) (CBMAB-E0375-FY)

-
Mouse Anti-FN1 Monoclonal Antibody (D6) (CBMAB-1240CQ)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Mouse Anti-BLK Recombinant Antibody (CBYY-0618) (CBMAB-0621-YY)

-
Mouse Anti-Acetyl SMC3 (K105/K106) Recombinant Antibody (V2-634053) (CBMAB-AP052LY)

-
Rabbit Anti-Acetyl-Histone H4 (Lys16) Recombinant Antibody (V2-623415) (CBMAB-CP1021-LY)

-
Mouse Anti-DISP2 Monoclonal Antibody (F66A4B1) (CBMAB-1112CQ)

-
Mouse Anti-CD33 Recombinant Antibody (P67.6) (CBMAB-C10189-LY)

-
Mouse Anti-CRTAM Recombinant Antibody (CBFYC-2235) (CBMAB-C2305-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot








