SOD2 Antibodies
Background
The SOD2 gene encodes manganese superoxide dismutase, a key antioxidant enzyme located in the mitochondrial matrix. This enzyme effectively eliminates reactive oxygen species produced by the mitochondrial electron transport chain by catalyzing the conversion of superoxide anions into hydrogen peroxide and oxygen, maintaining cellular REDOX homeostasis. As mitochondria are the energy factories of eukaryotic cells, SOD2 plays a core role in regulating cellular metabolism and preventing oxidative damage. Abnormal functions of SOD2 are closely related to aging, neurodegenerative diseases and the development of cancer. This gene was first cloned in 1989. Its three-dimensional structure analysis revealed a unique tetramer manganese cofactor binding mode, providing a molecular basis for understanding the antioxidant defense mechanism. It remains an important model in the field of oxidative stress research to this day.
Structure of SOD2
SOD2 is a homologous tetramer protein with a molecular weight of approximately 24 kDa, and its monomer is composed of 222 amino acids. This protein has highly conserved active centers among different species, but there are subtle differences in the overall molecular weight.
| Species | Human | Mouse | Rat | Bovine | Chicken |
| Molecular Weight (kDa) | 24.0 | 23.8 | 23.9 | 24.1 | 23.7 |
| Primary Structural Differences | Manganese ion coordination sites highly conservative | 93% homology to humans | The typical mitochondrial targeting sequence | The tetramer interface is highly conserved | The core domain remains intact |
Each monomer of SOD2 forms an active center through a manganese ion (Mn³⁺), and this metal cofactor directly participates in the catalytic process of superoxide disproportionation. The protein adopts a typical α -helical barrel structure framework, and the four subunits are assembled into a catalytic tetramer through conserved hydrophobic interactions. The histidine - aspartate - histidine triplet in its active center forms precise coordination with manganese ions. This structural feature is almost exactly the same in all eukaryotes, ensuring the high efficiency of electron transfer. The N-terminal of this enzyme also contains a characteristic mitochondrial localization signal peptide, which guides it to accurately enter the mitochondrial matrix to exert antioxidant functions.
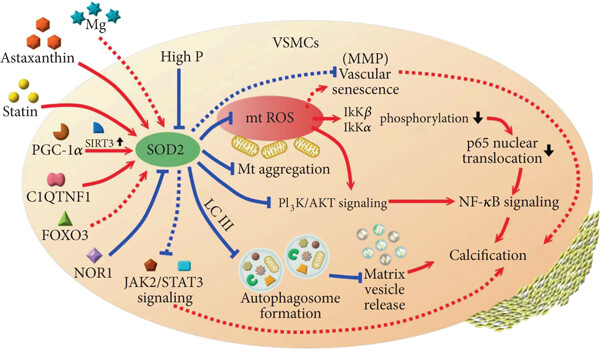 Fig. 1 SOD2 regulatory network in vascular smooth muscle cells.1
Fig. 1 SOD2 regulatory network in vascular smooth muscle cells.1
Key structural properties of SOD2:
- Conservative tetramer α -helical structure
- Hydrophobic core surrounded by manganese ion active center
- Manganese cofactors are responsible for the catalytic conversion of superoxides
Functions of SOD2
The main function of SOD2 is to eliminate superoxide anion radicals within mitochondria. In addition, this enzyme is also involved in the regulation of various physiological and pathological processes.
| Function | Description |
| Antioxidant defense | Catalyze the disproportionation reaction of superoxide anions to generate hydrogen peroxide and oxygen, forming the first line of antioxidant defense for cells. |
| Metabolic regulation | By regulating the level of reactive oxygen species to affect signaling pathways such as HIF-1α, it indirectly regulates cellular energy metabolism. |
| Cell protection | Reduce the damage to mitochondrial DNA and membrane structure caused by oxidative stress and maintain cell integrity. |
| Inflammatory regulation | Inhibit the activation of the NF-κB pathway mediated by reactive oxygen species and alleviate inflammatory responses. |
| Aging regulation | The active growth and decline with age, is closely related to tissue degeneration. |
The catalytic efficiency of SOD2 follows the kinetic characteristics of the Mie equation. Compared with SOD1 in the cytoplasm, it has a higher affinity for substrates, which is in line with its physiological environment in response to the transient high concentration of free radicals produced by mitochondrial electron leakage.
Applications of SOD2 and SOD2 Antibody in Literature
1. Alateyah, Nouralhuda, et al. "SOD2, a potential transcriptional target underpinning CD44-promoted breast cancer progression." Molecules 27.3 (2022): 811. https://doi.org/10.3390/molecules27030811
The article indicates that SOD2 is a key gene regulated by CD44 in breast cancer. It plays a role in promoting cancer by activating multiple signaling pathways, regulating the proliferation, invasion and angiogenesis of tumor cells. This article focuses on exploring its carcinogenic mechanism and the clinical transformation potential of its inhibitors.
2. Ibrahim, Nurul Khalida, et al. "SOD2 is a regulator of proteasomal degradation promoting an adaptive cellular starvation response." Cell reports 44.4 (2025). https://doi.org/10.1016/j.celrep.2025.115434
This study reveals a brand-new function of superoxide dismutase 2 (SOD2) when amino acids are scarce. SOD2 can regulate the proteasome degradation pathway through UBR1/2 ubiquitin ligase, providing amino acid sources for cancer cells and thereby maintaining their survival ability under metabolic stress.
3. Weyemi, Urbain, et al. "SOD2 deficiency promotes aging phenotypes in mouse skin." Aging (Albany NY) 4.2 (2012): 116. https://doi.org/10.18632/aging.100433
The article indicates that SOD2 is a key defense line against reactive oxygen species (ROS), responsible for eliminating superoxides within mitochondria. Studies have shown that the deficiency of SOD2 directly leads to thinning of the epidermis in mice, an increase in markers of cellular aging, and DNA damage. These phenomena are highly similar to normal aging characteristics, revealing the core role of SOD2 in inhibiting the aging process of the organism.
4. Tsai, You-Tien, et al. "Superoxide dismutase 2 (SOD2) in vascular calcification: a focus on vascular smooth muscle cells, calcification pathogenesis, and therapeutic strategies." Oxidative Medicine and Cellular Longevity 2021.1 (2021): 6675548. https://doi.org/10.1155/2021/6675548
The article indicates that SOD2 plays a core role in inhibiting vascular calcification by eliminating mitochondrial ROS in vascular smooth muscle cells. Its mechanisms also include regulating mitochondrial autophagy, inhibiting related signaling pathways and delaying vascular aging. Targeting SOD2 has emerged as a potential new strategy for treating vascular calcification.
5. Braun, Jessica L., et al. "Heterozygous SOD2 deletion selectively impairs SERCA function in the soleus of female mice." Physiological Reports 10.10 (2022): e15285. https://doi.org/10.14814/phy2.15285
The article indicates that the deficiency of SOD2 will exacerbate mitochondrial oxidative stress. Studies have shown that in mice with SOD2 heterozygous deletion, SERCA2a in soleus muscle undergoes specific tyrosine nitrification, resulting in a significant decrease in its calcium ion affinity. This is the first time that SOD2 deficiency selectively impairs the calcium pump function of specific muscles.
Creative Biolabs: SOD2 Antibodies for Research
Creative Biolabs specializes in the production of high-quality SOD2 antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Flow Cytometry, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom SOD2 Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our SOD2 antibodies, custom preparations, or technical support, contact us at email.
Reference
- Tsai, You-Tien, et al. "Superoxide dismutase 2 (SOD2) in vascular calcification: a focus on vascular smooth muscle cells, calcification pathogenesis, and therapeutic strategies." Oxidative Medicine and Cellular Longevity 2021.1 (2021): 6675548. https://doi.org/10.1155/2021/6675548
Anti-SOD2 antibodies
 Loading...
Loading...
Hot products 
-
Rabbit Anti-AP2M1 (Phosphorylated T156) Recombinant Antibody (D4F3) (PTM-CBMAB-0610LY)

-
Mouse Anti-F11R Recombinant Antibody (402) (CBMAB-0026-WJ)

-
Mouse Anti-ACTN4 Recombinant Antibody (V2-6075) (CBMAB-0020CQ)

-
Mouse Anti-ADGRL2 Recombinant Antibody (V2-58519) (CBMAB-L0166-YJ)

-
Mouse Anti-ADIPOR1 Recombinant Antibody (V2-179982) (CBMAB-A1368-YC)

-
Mouse Anti-BPGM Recombinant Antibody (CBYY-1806) (CBMAB-2155-YY)

-
Mouse Anti-AKR1C3 Recombinant Antibody (V2-12560) (CBMAB-1050-CN)

-
Rabbit Anti-CCN1 Recombinant Antibody (CBWJC-3580) (CBMAB-C4816WJ)

-
Mouse Anti-CCN2 Recombinant Antibody (CBFYC-2383) (CBMAB-C2456-FY)

-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Mouse Anti-CCL18 Recombinant Antibody (64507) (CBMAB-C7910-LY)

-
Rat Anti-EPO Recombinant Antibody (16) (CBMAB-E1578-FY)

-
Mouse Anti-ASTN1 Recombinant Antibody (H-9) (CBMAB-1154-CN)

-
Mouse Anti-DLC1 Recombinant Antibody (D1009) (CBMAB-D1009-YC)

-
Mouse Anti-FOXA3 Recombinant Antibody (2A9) (CBMAB-0377-YC)

-
Mouse Anti-ELAVL4 Recombinant Antibody (6B9) (CBMAB-1132-YC)

-
Mouse Anti-DMD Recombinant Antibody (D1190) (CBMAB-D1190-YC)

-
Rat Anti-EMCN Recombinant Antibody (28) (CBMAB-E0280-FY)

-
Mouse Anti-ATM Recombinant Antibody (2C1) (CBMAB-A3970-YC)

-
Mouse Anti-ALX1 Recombinant Antibody (96k) (CBMAB-C0616-FY)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






