ASMT Antibodies
Background
ASMT is a key methyltransferase, mainly found in the pineal gland and retina of vertebrates. This enzyme catalyzes the final step of melatonin synthesis by methylating N-acetylserotonin into melatonin, participating in the regulation of the body's circadian rhythm and antioxidant defense system. In 2004, the crystal structure of ASMT was first resolved, revealing its unique substrate binding pocket and the binding site of the methyl donor SAM. Studies have shown that ASMT gene polymorphisms are associated with various neuropsychiatric disorders, and abnormal expression levels can lead to disorders in melatonin synthesis. As the rate-limiting enzyme of the melatonin biosynthesis pathway, ASMT has become an important target for the study of biological clock regulation and neurodegenerative diseases.
Structure of ASMT
ASMT is a key enzyme with a molecular weight of approximately 28-32 kDa, and there are certain differences among different species:
| Species | Human | Mice | Bovine | Zebrafish | Arabidopsis thaliana |
| Molecular Weight (kDa) | 28.4 | 29.1 | 28.7 | 31.2 | 32.0 |
| Primary Structural Differences | Conserved catalytic domain | The C-end is relatively short | N-terminal modification | Contains additional domains | Plant-specific extension |
This enzyme is composed of approximately 250 to 300 amino acids and has a typical methyltransferase folding structure. Its active center contains a conserved S-adenosylmethionine (SAM) binding site and substrate recognition region. The key catalytic residues include histidine at position 156 (human ASMT) and aspartic acid at position 174, which jointly participate in the methyl transfer reaction. The protein surface has characteristic negatively charged regions that are responsible for binding to N-acetylserotonin. Asmts of different species show significant differences in C-terminal length and glycosylation sites, but the core catalytic structure is highly conserved.
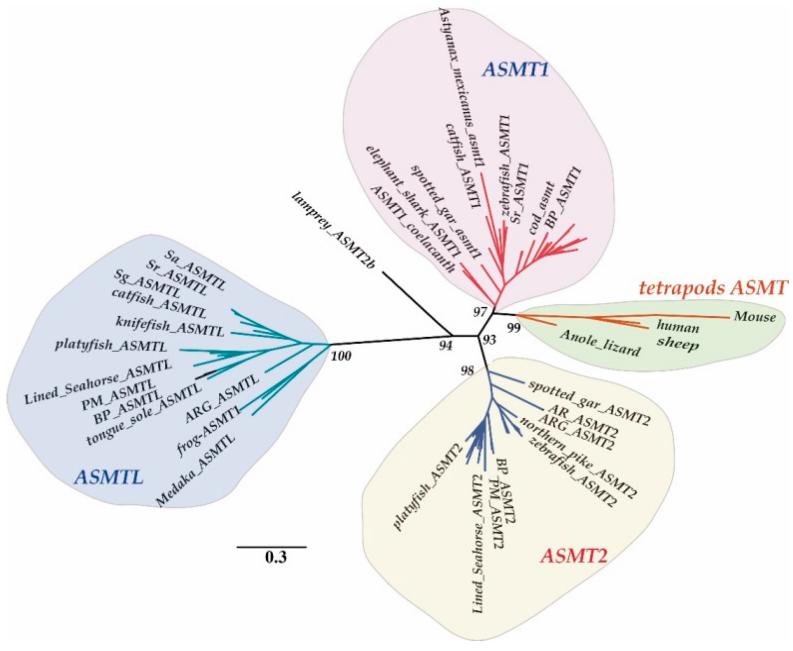 Fig. 1 Clustering of ASMT genes.1
Fig. 1 Clustering of ASMT genes.1
Key structural properties of ASMT:
- Typical methyltransferase folding structure (7 β -folds +8 α -helixes)
- Conservative S-adenosylmethionine (SAM) binding pocket
- Hydrophobic substrate binding chamber (containing N-acetylserotonin)
- The methyl transfer reaction is mediated by the key catalytic triad (His156-Asp174-Arg178)
- The C-terminal variable region determines species-specific functional differences
Functions of ASMT
The core function of ASMT is to catalyze the final step of melatonin synthesis and simultaneously participate in multiple physiological regulatory processes:
| Function | Description |
| Melatonin synthesis | Methylation of N-acetylserotonin into melatonin completes the synthesis of key hormones for the biological clock. |
| Regulation of circadian rhythm | Influencing the biological clock cycle (24-hour rhythm) by regulating melatonin levels. |
| Antioxidant defense | Mediated melatonin synthesis enhances the body's ability to eliminate free radicals. |
| Neuroprotection | Maintain the REDOX balance of the central nervous system. |
| Seasonal reproductive regulation | Regulating the reproductive cycle in photoperiodic sensitive animals. |
The catalytic efficiency of this enzyme is regulated by the light cycle, and its activity shows obvious diurnal fluctuations (the activity at night is 5 to 8 times higher than that during the day). Unlike most methyltransferases, ASMT is highly specific to the substrate N-acetylserotonin, with a Km value of approximately 120μM (in mammals). The latest research has found that ASMT may have non-classical functions in tissues outside the pineal gland, such as the retina and immune cells.
Applications of ASMT and ASMT Antibody in Literature
. Liu, Weina, et al. "ASMT determines gut microbiota and increases neurobehavioral adaptability to exercise in female mice." Communications Biology 6.1 (2023): 1126. https://doi.org/10.1038/s42003-023-05520-8
The article research indicates that ASMT antibody studies have found that female Asmt gene-mutated mice exhibit anxiety and depression behaviors and reduced motor adaptability due to impaired intestinal microbiota plasticity, while males do not have this phenotype. This suggests that ASMT enhances the neurobehavioral adaptability of females by maintaining microbiota plasticity.
. Zheng, Xiaodong, et al. "Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress." Scientific reports 7.1 (2017): 41236. https://doi.org/10.1038/srep41236
Research has found that the apple rootstock ASMT9 is located in the chloroplast and catalyzes the synthesis of melatonin. The melatonin level in Arabidopsis thaliana overexpressed with ASMT9 is elevated, significantly enhancing salt stress tolerance, reducing ROS accumulation and membrane lipid peroxidation, and improving photosynthetic efficiency, providing a new strategy for breeding stress-resistant crops.
. Zhang, Kai, et al. "A comparative genomic and transcriptomic survey provides novel insights into N-acetylserotonin methyltransferase (ASMT) in fish." Molecules 22.10 (2017): 1653. https://doi.org/10.3390/molecules22101653
Through comparative genomics and transcriptomics analysis, two subtypes of ASMT1 and ASMT2, as well as a novel ASMTL gene, were discovered in bony fish. The C-terminal of the ASMTL protein is homologous to ASMT. The three exhibit different tissue distributions and expression patterns, providing a new perspective for the study of the melatonin synthesis mechanism in fish.
. Ma, Kai, et al. "Walnut N-acetylserotonin methyltransferase gene family genome-wide identification and diverse functions characterization during flower bud development." Frontiers in plant science 13 (2022): 861043. https://doi.org/10.3389/fpls.2022.861043
The research revealed the evolutionary characteristics of the walnut JrASMT gene family through bioinformatics analysis and found that this family had undergone expansion and differentiation before the plant landed. A total of 46 JrASMT genes were identified, among which 4 had intraspecspecific/interspecific homology, 13 were differentially expressed during the sprout development period, and 4 were co-expressed with cell fate determination, providing new evidence for the analysis of the plant melatonin regulatory network.
. Cai, Guiqing, et al. "Multiplex ligation-dependent probe amplification for genetic screening in autism spectrum disorders: efficient identification of known microduplications and identification of a novel microduplication in ASMT." BMC Medical Genomics 1 (2008): 1-14. https://doi.org/10.1186/1755-8794-1-50
The study screened 279 autistic patients through MLPA technology and discovered novel microduplications in the 15q11-q13 region and partial duplications of the ASMT gene. The incidence of ASMT gene duplication in patients was significantly higher than that in the control group, suggesting that it may be involved in the pathogenesis of autism.
Creative Biolabs: ASMT Antibodies for Research
Creative Biolabs specializes in the production of high-quality ASMT antibodies for research and industrial applications. Our portfolio includes monoclonal antibodies tailored for ELISA, Western blot, immunohistochemistry, and other diagnostic methodologies.
- Custom ASMT Antibody Development: Tailor-made solutions to meet specific research requirements.
- Bulk Production: Large-scale antibody manufacturing for industry partners.
- Technical Support: Expert consultation for protocol optimization and troubleshooting.
- Aliquoting Services: Conveniently sized aliquots for long-term storage and consistent experimental outcomes.
For more details on our ASMT antibodies, custom preparations, or technical support, please contact us.
Reference
- Zhang, Kai, et al. "A comparative genomic and transcriptomic survey provides novel insights into N-acetylserotonin methyltransferase (ASMT) in fish." Molecules 22.10 (2017): 1653. https://doi.org/10.3390/molecules22101653
Anti-ASMT antibodies
 Loading...
Loading...
Hot products 
-
Mouse Anti-14-3-3 Pan Recombinant Antibody (V2-9272) (CBMAB-1181-LY)

-
Mouse Anti-BIRC5 Recombinant Antibody (6E4) (CBMAB-CP2646-LY)

-
Mouse Anti-ALB Recombinant Antibody (V2-55272) (CBMAB-H0819-FY)

-
Mouse Anti-AGK Recombinant Antibody (V2-258056) (CBMAB-M0989-FY)

-
Mouse Anti-APOE Recombinant Antibody (A1) (CBMAB-0078CQ)

-
Mouse Anti-AHCYL1 Recombinant Antibody (V2-180270) (CBMAB-A1703-YC)

-
Mouse Anti-C4B Recombinant Antibody (CBYY-C2996) (CBMAB-C4439-YY)

-
Mouse Anti-CD33 Recombinant Antibody (P67.6) (CBMAB-C10189-LY)

-
Mouse Anti-AKT1/AKT2/AKT3 (Phosphorylated T308, T309, T305) Recombinant Antibody (V2-443454) (PTM-CBMAB-0030YC)

-
Mouse Anti-ADIPOR2 Recombinant Antibody (V2-179983) (CBMAB-A1369-YC)

-
Mouse Anti-CCT6A/B Recombinant Antibody (CBXC-0168) (CBMAB-C5570-CQ)

-
Mouse Anti-CDK7 Recombinant Antibody (CBYY-C1783) (CBMAB-C3221-YY)

-
Mouse Anti-CRTAM Recombinant Antibody (CBFYC-2235) (CBMAB-C2305-FY)

-
Mouse Anti-BRCA2 Recombinant Antibody (CBYY-0790) (CBMAB-0793-YY)

-
Mouse Anti-DMPK Recombinant Antibody (CBYCD-324) (CBMAB-D1200-YC)

-
Rabbit Anti-ALOX5AP Recombinant Antibody (CBXF-1219) (CBMAB-F0750-CQ)

-
Rabbit Anti-ALDOA Recombinant Antibody (D73H4) (CBMAB-A2314-YC)

-
Mouse Anti-FAS2 Monoclonal Antibody (1D4) (CBMAB-0071-CN)

-
Mouse Anti-AAV9 Recombinant Antibody (V2-634029) (CBMAB-AP023LY)

-
Mouse Anti-FOXA3 Recombinant Antibody (2A9) (CBMAB-0377-YC)

- AActivation
- AGAgonist
- APApoptosis
- BBlocking
- BABioassay
- BIBioimaging
- CImmunohistochemistry-Frozen Sections
- CIChromatin Immunoprecipitation
- CTCytotoxicity
- CSCostimulation
- DDepletion
- DBDot Blot
- EELISA
- ECELISA(Cap)
- EDELISA(Det)
- ESELISpot
- EMElectron Microscopy
- FFlow Cytometry
- FNFunction Assay
- GSGel Supershift
- IInhibition
- IAEnzyme Immunoassay
- ICImmunocytochemistry
- IDImmunodiffusion
- IEImmunoelectrophoresis
- IFImmunofluorescence
- IGImmunochromatography
- IHImmunohistochemistry
- IMImmunomicroscopy
- IOImmunoassay
- IPImmunoprecipitation
- ISIntracellular Staining for Flow Cytometry
- LALuminex Assay
- LFLateral Flow Immunoassay
- MMicroarray
- MCMass Cytometry/CyTOF
- MDMeDIP
- MSElectrophoretic Mobility Shift Assay
- NNeutralization
- PImmunohistologyp-Paraffin Sections
- PAPeptide Array
- PEPeptide ELISA
- PLProximity Ligation Assay
- RRadioimmunoassay
- SStimulation
- SESandwich ELISA
- SHIn situ hybridization
- TCTissue Culture
- WBWestern Blot






